a. Use the data in Table 13.1-4 and the information that f G AgCl =
Question:
a. Use the data in Table 13.1-4 and the information that ΔfG°AgCl = −108.7 kJ/mol to predict the solubility product K°AgCl of silver chloride in water, and compare your predictions with the data given in Illustration 13.3-2.
b. Use the data in Table 13.1-4 and the information that ΔfG°TlCl = −186.02 kJ/mol to predict the solubility product K°TlCl of thallium chloride in water, and compare your predictions with the data given in Illustration 13.3-2.
Table 13.1-4
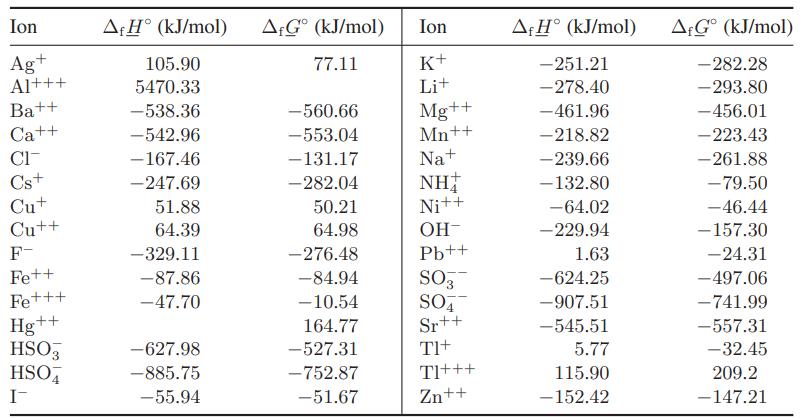
Illustration 13.3-2.
The solubility of silver chloride in water at 25°C is 1.273 × 10−5 kmol/m3, and that of thallium chloride is 0.144 kmol/m3. Estimate the simultaneous solubility of AgCl and TlCl in water.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





