At an appropriate temperature, a larger alkane will crack to former a smaller alkane and an olefin.
Question:
At an appropriate temperature, a larger alkane will “crack” to former a smaller alkane and an olefin. One example is the cracking of propane to form methane and ethylene
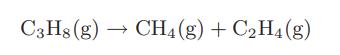
a. Calculate the equilibrium compositions that would result at 298.15 K and a total pressure of 1 bar by starting with pure propane.
b. Assuming that the standard state heat of reaction for this reaction is independent of temperature, repeat the calculation above at 650 K.
c. Repeat the calculation of Part b for a total pressure of 10 bar assuming that an ideal gas mixture is formed at this high temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





