At high temperatures phosphine (PH 3 ) dissociates into phosphorus and hydrogen by the following reaction: for
Question:
At high temperatures phosphine (PH3) dissociates into phosphorus and hydrogen by the following reaction:
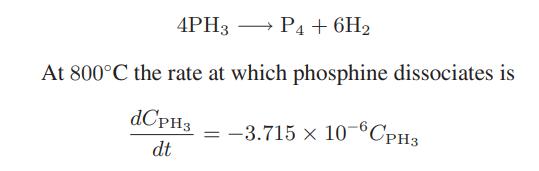
for t in seconds. The reaction occurs in a constantvolume, 2-L vessel, and the initial concentration of phosphine is 5 kmol/m3
a. If 3 mol of the phosphine reacts, how much phosphorus and hydrogen are produced?
b. Develop expressions for the number of moles of phosphine, phosphorus, and hydrogen present at any time, and determine how long it would take for 3 mol of phosphine to have reacted.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





