Question: Redo Problem 13.46 using Aspen Plus. Problem 13.46 While ethanol can be made by biological fermentation, for large-scale production the following nonbiological reaction starting with
Redo Problem 13.46 using Aspen Plus.
Problem 13.46
While ethanol can be made by biological fermentation, for large-scale production the following nonbiological reaction starting with ethylene and water can be used instead:
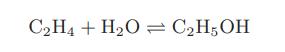
If stoichiometric amounts of ethylene and water are used, compute the equilibrium constant and the equilibrium extent of reaction at
a. 1 bar and 25°C, assuming the liquid solution is ideal
b. Repeat the calculation assuming a nonideal liquid solution if formed.
C2H4 + HO CH5OH
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
a Assuming the liquid solution is ideal To compute the equilibrium constant and the equilibrium extent of reaction at 1 bar and 25C using Aspen Plus i ... View full answer

Get step-by-step solutions from verified subject matter experts


