Silver, when exposed to air, tarnishes. The following reactions have been proposed for this tarnishing: The following
Question:
Silver, when exposed to air, tarnishes. The following reactions have been proposed for this tarnishing:
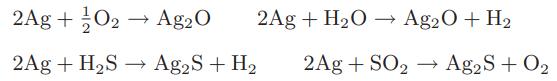
The following data are available:
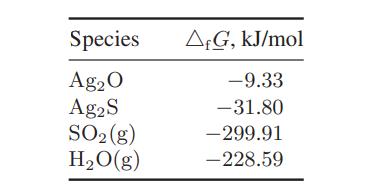
Air can be assumed to contain 0.5 ppm H2, 0.03 ppm (80 mg/m3) SO2, and 0.1 H2S mg/m3 and water at a partial pressure of 2.0 kPa.
Which of these reactions are likely to occur at normal conditions and result in the tarnishing of silver?
Transcribed Image Text:
2Ag + O2 → Ag2O 2Ag + H₂S → Ag2S + H₂ 2Ag + H₂O → Ag20 + H₂ 2Ag + SO₂ → Ag2S + O₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine which reactions are likely to occur and result in the tarnishing of silver we can compa...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Rod AB of length 13 in. is connected by ball-and-socket joints to collars A and B, which slide along the two rods shown. Knowing that collar B moves toward point D at a constant speed of 36 in./s,...
-
A cyclist uses a stationary trainer during the winter to keep in shape. Knowing that she pushes down on her pedal with a velocity of 26 in./s that increases at a rate of 2 in./s 2 , determine the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Show that is one-to-one and find f(x) = J2 1 + 1 dt
-
What role do performance reports play with respect to the control function?
-
The assets and liabilities of Impeccable Travel Service at November 30, 2010, the end of the current year, and its revenue and expenses for the year are listed below. The capital of the owner, Charly...
-
Gold-on-gold nuclear collisions at the Relativistic Heavy Ion Collider (RHIC) at the Brookhaven National Laboratory create a quark-gluon plasma with an energy density of about \(4 \mathrm{GeV} /...
-
Francisco Company uses the columnar cash journals illustrated in the textbook. In April, the following selected cash transactions occurred. 1. Made a refund to a customer as an allowance for damaged...
-
17.4 Suppose that, for R R. Let {c} be the Laurent coefficients of in the annulus R < || < S. Prove, by considering the expansions of g and h, that 9(z) = c + cnzn (|z|
-
By catalytic dehydrogenation, 1-butene can be produced from n-butane, C 4 H 10 = C 4 H 8 + H 2 However, 1-butene may also be dehydrogenated to form 1,3-butadiene, C 4 H 8 = C 4 H 6 + H 2 Compute the...
-
Styrene can be hydrogenated to ethyl benzene at moderate conditions in both the liquid phase and the gas phase. Calculate the equilibrium compositions in the vapor and liquid phases of hydrogen,...
-
In Exercises 31 and 32, identify the theorem or postulate you would use to prove the triangles congruent. A A B S D E C D F
-
Jamal Company began operations on July 1, 2013. Information from job cost sheets shows the following: Job 102 was completed in July. Job 100 was completed in August, and Jobs 101 and 103 were...
-
Go to the home page of a large corporate and find its latest financial or annual reports. Look for key words in the income statement that appear in this chapter, such as sales, gross profits, and net...
-
Nagham Manufacturing is a small manufacturer that uses machine hours as its activity base for assigning overhead costs to jobs. The company estimated the following amounts for 2013 for the company...
-
The following direct labor data pertain to the operations of Hamad Manufacturing Company for the month of November: The standard cost card shows that 2.5 hours are required to complete one unit of...
-
Farid Manufacturing Company uses a job order cost accounting system and keeps perpetual inventory records. Prepare journal entries to record the following transactions during the month of June. June...
-
Assume that the partners of Exercise 12-5 agreed to share net income and loss by granting annual salary allowances of $50,000 to Kramer and $40,000 to Knox, 10% interest allowances on their...
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
Replace the force of F = 80 N acting on the pipe assembly by an equivalent resultant force and couple moment at point A. A 400 mm B 300 mm 200 mm 200 mm 250 mm 40 30 F = 80 N
-
Replace the loading by an equivalent resultant force and couple moment acting at point O. 50 Ib/ft -9 ft 9 ft 50 Ib/ft
-
Replace the loading by an equivalent resultant force and specify its location on the beam, measured from point A. 5 kN/m 2 kN/m 4 m- 2 m
-
Part One: Electrons stick to Scotch Magic Tape and today you will estimate the number of electrons stuck to the surface of a piece of tape. Here are a few suggestions to get you started, but you will...
-
A ship loaded with cargo displaces 6,799 cubic meters (m3) of water. Determine the combined weight of the ship and its cargo, in mega-Newtons (MN). Assume the density of water is 997 kg/m3. Hint...
-
A mass (m=1.00 kg) attached to a rigid rod of negligible mass swings in a vertical circle (R=1.00 m) with a frequency of 15.0 Hz. At t = 0, the mass is located at 1.00 m, 30.0o . a) Find the maximum...

Study smarter with the SolutionInn App


