Steam is continuously expanded from a pressure of 25 bar and 300C to 1 bar through a
Question:
Steam is continuously expanded from a pressure of 25 bar and 300°C to 1 bar through a Joule-Thomson expansion valve. Calculate the final temperature and the entropy generated per kilogram of steam using
a. The ideal gas law
b. The van der Waals equation of state
c. The Peng-Robinson equation of state
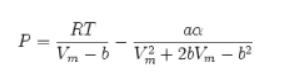
d. The steam tables
Transcribed Image Text:
P = RT aa Vm-b V2+2bVm – b²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The ideal gas law The ideal gas law is a simple equation of state that relates the pressurevolumeand temperature of a gas PV nRT where P is the pressure of the gas V is the volume of the gas n is the ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Redo Problem 6.31 using Aspen Plus Problem 6.31 Steam is continuously expanded from a pressure of 25 bar and 300C to 1 bar through a Joule-Thomson expansion valve. Calculate the final temperature and...
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
Suppose that in a wave of pessimism, housing prices fall by 10% across the entire economy. a. Has the stock of real assets of the economy changed? b. Are individuals less wealthy? c. Can you...
-
A conceived a secret process for the continuous freeze-drying of foodstuffs and related products and constructed a small pilot plant that practiced the process. A, however, lacked the financing...
-
Minesweeper, the well-known computer game, is closely related to the wumpus world. A minesweeper world is a rectangular grid of N squares with M invisible mines scattered among them. Any square may...
-
In December 2008, Jason Garcia signed a motor vehicle sales contract with Mac Haik Dodge Chrysler Jeep, a dealer. In the contract, Garcia agreed to purchase a 2009 Dodge Ram 1500. The contract...
-
The following information pertains to Worthy Video Company. 1. Cash balance per bank, July 31, $7,293. 2. July bank service charge not recorded by the depositor $28. 3. Cash balance per books, July...
-
Estimated total machine-hours used Estimated total fixed manufacturing overhead Estimated variable manufacturing overhead per machine-hour Molding 2,500 $ 12,000 $ 2.20 Fabrication 1,500 $ 16,200 $...
-
In the calculation of thermodynamic properties, it is convenient to have the following partial derivatives: where Z = (P V /RT) is the compressibility factor. Develop expressions for these two...
-
For real gases the Joule-Thomson coefficient is greater than zero at low temperatures and less than zero at high temperatures. The temperature at which is equal to zero at a given pressure is called...
-
Walsh Equipment Company made the following purchases of debt securities during 2012. All are classified as held-to-maturity, and all pay interest semiannually. Required: 1. Prepare journal entries...
-
For each of the types of intelligence required for a Transformational leader, give a behaviour that would indicate the person has this intelligence. Cognitive intelligence - Emotional intelligence -...
-
Kylie is an 18-year-old female weighing 110 lbs. (50 kg), and 5 feet 8 inches in height. She is a competitive track runner and has excelled in both the 400- and 800-meter dash (high-intensity,...
-
How do individuals negotiate their sense of identity within the context of intersecting social, cultural, and political frameworks, and what role does this negotiation play in shaping collective...
-
How have traditional gender role expectations influenced the perceived behavioral norms for women and men in the United States, particularly emphasizing a patriarchal orientation where societal...
-
The California Wood Harvesting Company has been clear-cutting for over 50 years in Northern California. The company has always complied with governmental regulations and actively replants the areas...
-
Offhaus Manufacturing produces office supplies, but outsources the delivery of its products to third party carriers. Offhaus ships to 20 cities from its Dayton, Ohio, manufacturing facility and has...
-
Assume today is the 21st of February. Using the information below, FT Extract, answer the following questions (parts i and ii). You work for a US company that is due to receive 250 million in June...
-
A tank having vertical sides and a bottom area of 100 ft 2 is used to store water. To fill the tank, water is pumped into the top at the rate given in the following table. Use Simulink to solve for...
-
Construct a Simulink model to plot the solution of the following equations for 0 t 2 where f(t) = 3t. Use the Ramp block in the Sources library. ij = -6x1 + 4x2 i2 = 5x - 7x, +f(t)
-
Construct a Simulink model to plot the solution of the following equations for 0 t 3 where f 1 (t) is a step function of height 3 starting at t = 0 and f 2 (t) is a step function of height -3...
-
Concussions and brain size. What is the effect of concussions on the brain? Researchers measured the brain sizes (hippocampal volume in microliters) of 25 collegiate football players with a history...
-
Many aspects of math can easily be seen in everyday life and other arent as visible but are still present.By learning about Expected Value, Correlation and Regression. Reflect and describe a time...
-
Each supplier has a limited capacity in terms of the total number of components it can supply. However, as long as Edwards provides sufficient advance orders, each supplier can devote its capacity to...

Study smarter with the SolutionInn App


