The following data are available for carbon tetrachloride: a. Compute the heat of vaporization of carbon tetrachloride
Question:
The following data are available for carbon tetrachloride:
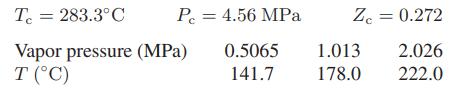
a. Compute the heat of vaporization of carbon tetrachloride at 200°C using only these data.
b. Derive the following expression, which can be used to compute the heat of vaporization from the principle of corresponding states:
![H - HIG H HIG [(7) -("7").] Te Te AvapH = Tc sat vap sat liq](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/0/3/1/35465546b7a0c9411700031350139.jpg)
c. Compute the heat of vaporization of carbon tetrachloride at 200°C using the principle of corresponding states.
d. Comment on the reasons for the difference between the heats of vaporization computed in parts (a) and (c) and suggest a way to correct the results to improve the agreement.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





