The following data are available for the thermodynamic properties of graphite and diamond: Assuming that the entropies
Question:
The following data are available for the thermodynamic properties of graphite and diamond:
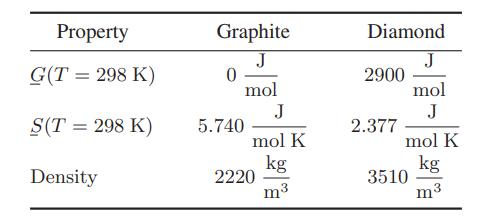
Assuming that the entropies and densities are approximately independent of temperature and pressure, determine the range of conditions for which diamonds can be produced from graphite. (The procedure proposed would be to hold the graphite at high temperatures and pressures until diamonds formed, followed by rapid cooling and depressurizing so that the diamonds can not revert to graphite.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





