The following vapor-liquid equilibrium data are available for methyl ethyl ketone: Heat of vaporization at 75C: 31
Question:
The following vapor-liquid equilibrium data are available for methyl ethyl ketone:
Heat of vaporization at 75°C: 31 602 J/mol Molar volume of saturated liquid at 75°C: 9.65×10−2 m3 /kmol
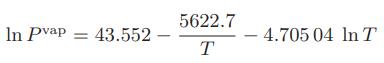
where P is the vapor pressure in bar and T is in K. Assuming the saturated vapor obeys the volume explicit form of the virial equation,
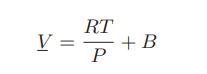
Calculate the second virial coefficient, B, for methyl ethyl ketone at 75°C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





