A (60 mathrm{vol} %) tributyl phosphate (TBP) in kerosene solvent extracts (mathrm{Zr}left(mathrm{NO}_{3} ight)_{4}) from an aqueous solution.
Question:
A \(60 \mathrm{vol} \%\) tributyl phosphate (TBP) in kerosene solvent extracts \(\mathrm{Zr}\left(\mathrm{NO}_{3}\right)_{4}\) from an aqueous solution. Entering solvent is recycled from a solvent recovery system and contains \(0.008 \mathrm{~mol} \mathrm{Zr}\left(\mathrm{NO}_{3}\right)_{4} / \mathrm{L}\). Aqueous feed contains \(0.10 \mathrm{~mol} \mathrm{Zr}\left(\mathrm{NO}_{3}\right)_{4} / \mathrm{L}\) and outlet aqueous solution should contain \(0.008 \mathrm{~mol} \mathrm{Zr}\left(\mathrm{NO}_{3}\right)_{4} / \mathrm{L}\). Feed rate of aqueous solution is \(200 \mathrm{~L} / \mathrm{h}\).
a. Find minimum entering solvent rate \(\mathrm{F}_{\text {solvent,min }}\) in \(\mathrm{L} / \mathrm{h}\).
b. If \(\mathrm{F}_{\text {solven }}=1.4 \mathrm{~F}_{\text {solvent,min }}\), find number of equilibrium stages required.
c. Calculate mole fraction and mass fraction of \(\mathrm{Zr}\left(\mathrm{NO}_{3}\right)_{4}\) in feed and determine if system is dilute. Assume density of feed is \(1.0 \mathrm{~g} / \mathrm{ml}\).
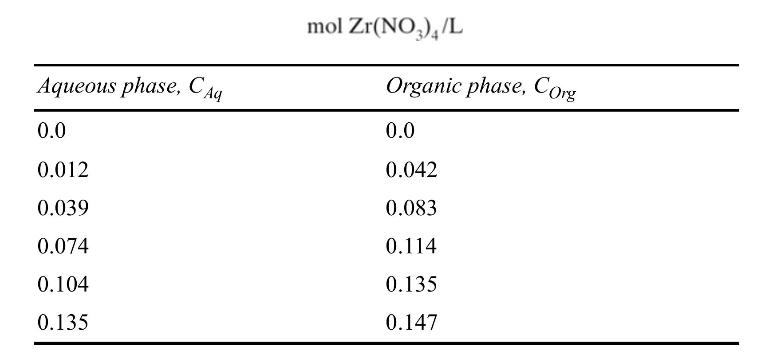
Assume that aqueous and organic phases are completely immiscible and that densities of the two phases are both constant (volumetric flow rates are constant). For zirconium nitrate, \(\mathrm{Zr}\left(\mathrm{NO}_{3}\right)_{4}\), equilibrium data are listed in the following table for \(60 \mathrm{vol} \% \mathrm{TBP}\) in kerosene (Benedict and Pigford, 1957):
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





