Question: A distillation column is separating methanol from water. The column has a total condenser that subcools the reflux so that 1 mole of vapor is
A distillation column is separating methanol from water. The column has a total condenser that subcools the reflux so that 1 mole of vapor is condensed in the column for each 3 moles of reflux. \(\mathrm{L}_{0} / \mathrm{D}=3\). A liquid side stream is withdrawn from the second stage below the condenser. This side stream is vaporized to a saturated vapor and then mixed with the feed and input on stage 4. The side withdrawal rate is \(\mathrm{S}=500 \mathrm{kmol} / \mathrm{h}\). The fresh feed is a saturated vapor that is 48 \(\mathrm{mol} \%\) methanol. Fresh feed rate is \(\mathrm{F}=1000 \mathrm{kmol} / \mathrm{h}\). A total reboiler is used, which produces a saturated vapor boilup. Distillate is \(92 \mathrm{~mol} \%\) methanol, and bottoms is \(4 \mathrm{~mol} \%\) methanol. Assume CMO. VLE data are in Table 2-8. Find
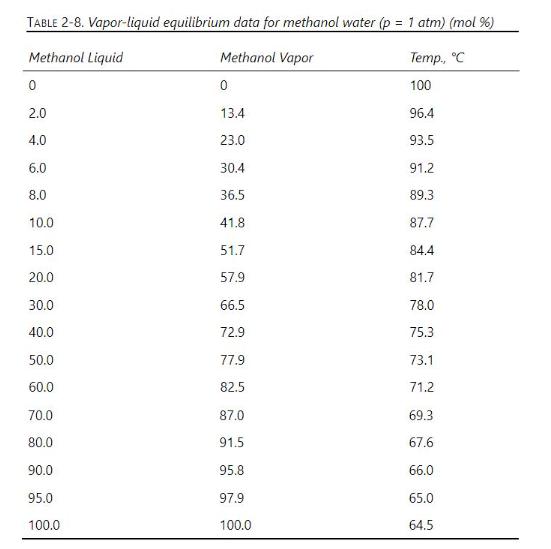
a. The total number of equilibrium stages required.
b. The value of \(\sqrt{ } / \mathrm{B}\).
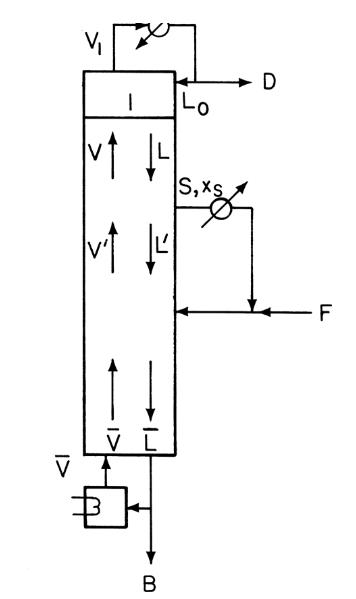
TABLE 2-8. Vapor-liquid equilibrium data for methanol water (p = 1 atm) (mol %) Methanol Liquid Methanol Vapor Temp., C 0 0 100 2.0 13.4 96.4 4.0 23.0 93.5 6.0 30.4 91.2 8.0 36.5 89.3 10.0 41.8 87.7 15.0 51.7 84.4 20.0 57.9 81.7 30.0 66.5 78.0 40.0 72.9 75.3 50.0 77.9 73.1 60.0 82.5 71.2 70.0 87.0 69.3 80.0 91.5 67.6 90.0 95.8 66.0 95.0 97.9 65.0 100.0 100.0 64.5
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


