A mixture of acetone and ethanol is distilled at (1.0 mathrm{~atm}) in a distillation column with a
Question:
A mixture of acetone and ethanol is distilled at \(1.0 \mathrm{~atm}\) in a distillation column with a total condenser and a partial reboiler. We desire a distillate that is 0.999 mole fraction acetone and a bottoms that is 0.0013 mole fraction acetone. Feed is \(40 \mathrm{~mol} \mathrm{\%} \mathrm{acetone,} \mathrm{it} \mathrm{is} \mathrm{a} \mathrm{two-}\) phase mixture that is \(5 / 6\) liquid, and feed flow rate is \(50 \mathrm{~mol} / \mathrm{h}\). Data are in Problem 4.D7.
Problem 4.D7
Your boss wants some idea of how expensive it will be to distill 155.0 \(\mathrm{kmol} / \mathrm{h}\) of a saturated liquid feed that is \(5.0 \mathrm{~mol} \%\) methane, \(10.0 \mathrm{~mol} \%\) ethane, \(15.0 \mathrm{~mol} \%\) n-butane, \(22.0 \mathrm{~mol} \% \mathrm{n}\)-pentane, \(22.0 \mathrm{~mol} \% \mathrm{n}-\) hexane, and \(26.0 \mathrm{~mol} \% \mathrm{n}\)-heptane. Column pressure is \(700.0 \mathrm{kPa}\). The column has a partial condenser and a partial reboiler. We want to recover \(99.0 \%\) of the \(n\)-butane in the distillate and \(98.3 \%\) of the \(n-\) pentane in the bottoms. Do the calculations of the \(\mathrm{K}\) values either from the DePriester chart or from Eq. (2-28).
Equation (2-28)
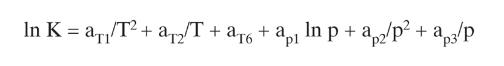
a. Determine the relative volatility near the top of the column, near the bottom of the column, and near the intersection of the feed line and the equilibrium curve. Calculate the appropriate average relative volatility.
b. Use the Fenske equation to determine the number of equilibrium contacts at total reflux.
c. Assume \(\mathrm{CMO}\) is valid and calculate the value of (L/D) \()_{\min }\) from the McCabe-Thiele diagram.
d. Use the Gilliland correlation (or the Davis equation) to estimate the number of stages if \(\mathrm{L} / \mathrm{D}=1.05(\mathrm{~L} / \mathrm{D})_{\min }\).
e. Estimate the optimum feed stage location.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





