A need arises in a laboratory to conduct experiments in an atmosphere of phosgene, which is highly
Question:
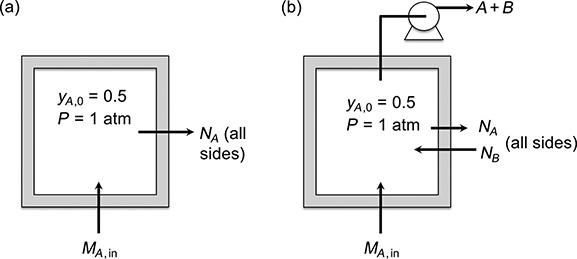
A need arises in a laboratory to conduct experiments in an atmosphere of phosgene, which is highly toxic. The experiments will be conducted inside a plywood box designed to contain a mixture of phosgene
(A) and air (B) having a constant 0.50 mole fraction of phosgene as shown in Fig. P.11.2. Because of the porosity of the plywood, phosgene will diffuse across the walls of the box. In order to reduce the leakage of phosgene, it is proposed to connect a small gas pump to the box to continuously pump out the air-phosgene–mixture while simultaneously feeding pure phosgene to the box to keep a constant mole fraction. The pump will create a slow flow of air into the box through the plywood, thereby reducing the leakage of phosgene. The pump will discharge into the laboratory hood exhaust system.
(a) Determine the leakage of phosgene by diffusion through the plywood if no pumping occurs. Note that phosgene will have to be added to the box continuously to keep a constant mole fraction in the box.
(b) Determine the inward flow rate of air and the pumping rate required to reduce the phosgene leakage to one-tenth of its value with no pumping.
(c) Determine the rate at which pure phosgene must be supplied to the box to continuously replace the phosgene that still diffuses out plus the phosgene that is pumped out along with air.
Data: The box is a cube with 60-cm-long sides and a wall thickness of 0.5 cm. The temperature is 25°C and the pressure both inside and outside the box is 1 atm. The phosgene mole fraction outside the box may be taken to be 0. The effective diffusivity of phosgene in the plywood is 0.011 cm2/s.
FIGURE P.11.2:
Step by Step Answer:

Heat And Mass Transfer For Chemical Engineers Principles And Applications
ISBN: 9781264266678
1st Edition
Authors: Giorgio Carta





