A saturated vapor feed at (1000.0 mathrm{kmol} / mathrm{h}) of methanol ((5.0 mathrm{~mol} %)) and water ((95.0
Question:
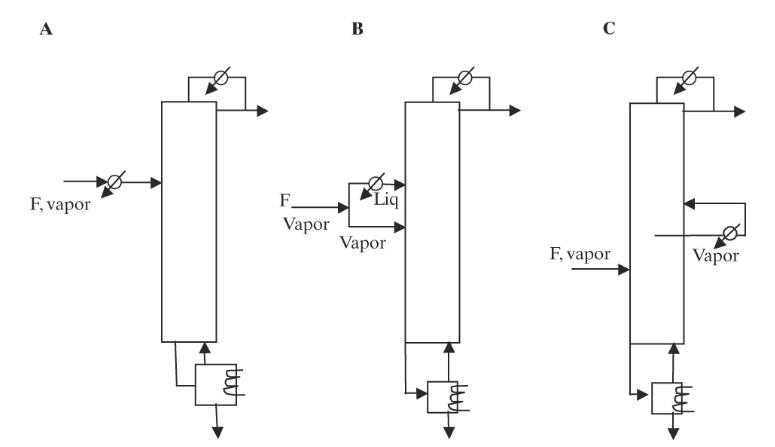
A saturated vapor feed at \(1000.0 \mathrm{kmol} / \mathrm{h}\) of methanol \((5.0 \mathrm{~mol} \%)\) and water \((95.0 \mathrm{~mol} \%)\) is fed to a distillation column with 18 stages plus a kettle reboiler and a total condenser \((\mathrm{N}=20\) in Aspen Plus notation). Use the NRTL VLE correlation. Operate at \(80 \%\) of flooding using Fair's diameter calculation method and tray spacing \(=0.4572 \mathrm{~m}\). Use an external reflux ratio of 24 . Pressure is \(1.0 \mathrm{~atm}\). Calculate the optimum feed plate location, product purities, \(\mathrm{Q}_{\mathrm{c}}\) and \(\mathrm{Q}_{\mathrm{R}}\), and the column diameters at different locations for the base case. Then determine which of the methods in Figure 10-17 will do the best job of balancing the diameters and reducing the column volume with constant product purities, \(Q_{c}\) and \(Q_{R}\). Report the results for your system with the largest volume reduction.
Figure 10-17
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





