Question: Do the matrix for (n)-butane for Problem 6.D2. Problem 6.D2 A distillation column operating at (5.0 mathrm{~atm}) has a total condenser and a partial reboiler.
Do the matrix for \(n\)-butane for Problem 6.D2.
Problem 6.D2
A distillation column operating at \(5.0 \mathrm{~atm}\) has a total condenser and a partial reboiler. The saturated liquid feed flow rate is \(1000.0 \mathrm{kmol} / \mathrm{h}\). Feed is \(8.0 \mathrm{~mol} \%\) ethane, \(33.0 \mathrm{~mol} \%\) propane, \(49.0 \mathrm{~mol} \% \mathrm{n}\)-butane, and \(10.0 \mathrm{~mol} \% \mathrm{n}\)-pentane. The column has four equilibrium stages and the partial reboiler, which is an equilibrium contact. The feed is on second stage below the total condenser. The reflux ratio \(\mathrm{L}_{0} / \mathrm{D}=2.5\). Distillate flow rate \(\mathrm{D}=410 \mathrm{kmol} / \mathrm{h}\). Develop the mass balance and equilibrium matrix (Eq. 6-13) with numerical values for each element \(\left(A_{j}, B_{j}, C_{j}\right.\), and \(\left.D_{j}\right)\). Do this for your first guess: \(T_{j}=\) bubble-point temperature of feed (use the same temperature for all stages); \(\mathrm{K}\) values are from the DePriester chart; and \(\mathrm{L}\) and \(\mathrm{V}\) are CMO values. Do the matrix for propane only.
Equation 6-13
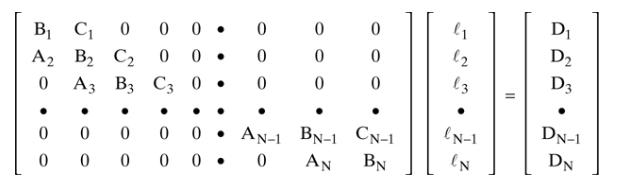
(1 12 13 D D2 D3 . . BN-1 CN-1 (N-1 DN-1 AN BN IN DN 000 000 . B C C 0 A2 B2 0 00. B C2 0 0. A3 B3 C3 0. 0 0 0 0 0 0 0 0 0 0AN-1 0000 .
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


