The reaction acetic acid (A) + butanol (B)ester + water was studied by Leyes and Othmer, who
Question:
The reaction acetic acid (A) + butanol (B)→ester + water was studied by Leyes and Othmer, who found the data in Table 6.P4 at 100◦C in the presence of 0.03 percent sulfuric acid. The initial concentrations were 0.2327 mol acetic acid/100 g of solution and 1.583 mol butanol/100 g of solution. Leyes and Othmer claimed that the reaction is second order in acetic acid and zero order in butanol up to 75 percent conversion; i.e., r = kc2A . Do the data support this contention? Can you distinguish this relation from r = kcAcB with the data given here? (You may assume that the density of the solution is constant over the course of the reaction with a value of approximately 750 kg/m3. Why is this assumption permissible?)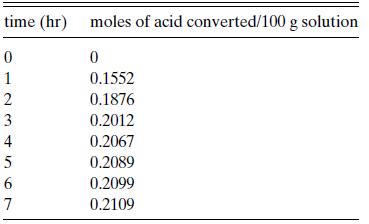
time (hr) moles of acid converted/100 g solution 0 1 2 3 4 5 6 69 7 0 0.1552 0.1876 0.2012 0.2067 0.2089 0.2099 0.2109
Step by Step Answer:
Well utilise the reactions ra...View the full answer



Students also viewed these Engineering questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
After deducting capital allowances for the year to 31 December 2019, the tax written down values of a company's plant and machinery were as follows: Calculate the capital allowances available for the...
-
What is the purpose of the client's performance measurement system? How might that system be useful to the auditor? Give examples of key performance indicators for the following businesses: (1) A...
-
What does the slope of the aggregate expenditure line equal? How is the slope of the aggregate expenditure line related to the slope of the consumption function?
-
Use technology to construct a histogram for the frequency distribution in Example 2. Data from Example 2 Using the frequency distribution constructed in Example 1, find the midpoint, relative...
-
Listed below are items that are treated differently for accounting purposes than they are for taxpurposes. Indicate whether the items are permanent differences or temporary differences. For temporary...
-
Two Firefighter/Paramedics who work on opposite shifts have an ongoing personality conflict. One of the paramedics Bill Jones (a 40 year old Army veteran) has come to the supervisor on several...
-
The saponification of the ester propargyl acetate, with base (B, OH) was studied by Myers, Collett, and Lazzell using a conductivity technique to follow the course of the reaction. Conductivity,...
-
The hydrolysis of acetic anhydride in excess water to form acetic acid, (CH 3 CO) 2 O + H 2 O2CH 3 COOH, was studied by Elridge and Piret, who found that the rate at 15 C is first order in...
-
Before working this problem, review Conceptual Example 14. A pellet gun is fired straight downward from the edge of a cliff that is 15 m above the ground. The pellet strikes the ground with a speed...
-
Below are two of Comfy Home's T-accounts reflecting recent journal entries. CASH ACCOUNT Debit Credit Beginning $29,000 Balance 1/15/2020 $25,000 Ending $4,000 Balance ACCOUNTS PAYABLE Debit Credit...
-
Marginal Revenue for an Apartment Complex Lynbrook West, an apartment complex, has 100 two-bedroom units. The monthly profit (in dollars) realized from renting x apartments is represented by the...
-
7. The following information is from the noncurrent liabilities and owners' equity portions of Colony LLC's balance sheet: December 31 2020 2019 Bonds payable $ 410,000 $330,000 Common stock 800,000...
-
In its first year of operations, Melissa Corp, earned $ 5 8 0 0 0 in service revenue. Of that amount, $ 9 5 0 0 was on account and the remainder, $ 4 8 5 0 0 , was collected in cash from customers....
-
Spring Appliances received an invoice dated August 1 8 with terms 2 / 1 0 E . O . M . for the items listed below. 6 refrigerators at $ 1 0 3 0 each less 3 0 % and 5 % 3 dishwashers at $ 7 8 4 each...
-
Access to an organizations funds can be gained through counterfeiting the organizations check stock. What types of controls would help detect a counterfeit check?
-
Propose a reasonable mechanism for the following reaction. OH
-
We want to bond together two slabs of a solid material, each with a thickness of 1.5 cm, to form a laminate by using a thin layer of a thermosetting glue, which fuses and forms a bond between the two...
-
An air-cooled utility engine needs to dissipate 2,000 W of heat in order to operate at a steady temperature of 150C when the ambient temperature is 25C. The engine block can be represented as a...
-
Consider a straight fin with the trapezoidal shape sketched in Fig. P.4.9. Assuming that the temperature in the fin is only a function of the axial coordinate x (i.e., it is uniform at each vertical...
-
The trial balance of Martinez Ltd. at December 31, 2023, follows: Cash Sales revenue Debits Credits $345,000 $11,434,000 FV-NI investments (at fair value) Cost of goods sold Bond investment at...
-
Williams Company uses a periodic inventory system. The following are Inventory transactions for the month of March: 3/1 Beginning Inventory 5,000 units at $2 3/7 3/16 Purchase 2,500 units at $3...
-
On February 1, 2024, Sanyal Motor Products issued 8% bonds, dated February 1, with a face amount of $90 million. The bonds mature on January 31, 2028 (four years). The market yield for bonds of...

Study smarter with the SolutionInn App


