We flash distill a mixture of methane and n-butane at (mathrm{p}_{text {drum }}=6.0) bar. a. Use the
Question:
We flash distill a mixture of methane and n-butane at \(\mathrm{p}_{\text {drum }}=6.0\) bar.
a. Use the DePriester charts or Eq. (2-28). For several temperatures from \(\mathrm{T}=\) \(-60^{\circ} \mathrm{C}\) to \(\mathrm{T}=50^{\circ} \mathrm{C}\), calculate the values of \(\mathrm{y}\) and \(\mathrm{x}\) in equilibrium. Plot equilibrium curve of \(y_{\text {methane }}\) versus \(x_{\text {methane }}\) and plot temperature \(T\) as the ordinate versus \(\mathrm{x}_{\text {methane }}\) (abscissa). (Note: It is convenient to plot \(\mathrm{T}\) versus \(\mathrm{x}\) on the \(\mathrm{y}\)-x graph placing the \(\mathrm{T}\) scale on the right side of the diagram.)
Equations (2-28)
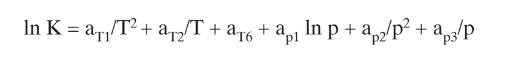
b. If the feed rate is \(250.0 \mathrm{kmol} / \mathrm{h}\), feed is \(40.0 \mathrm{~mol} \%\) methane, \(\mathrm{V} / \mathrm{F}=0.70\), and \(\mathrm{p}_{\text {drum }}=6.0\) bar, find \(\mathrm{x}, \mathrm{y}, \mathrm{T}_{\text {drum }}, \mathrm{L}\), and \(\mathrm{V}\).
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat




