Question: A chemist needs to prepare a solution buffered at pH 4.30 using one of the following acids (and its sodium salt): Calculate the ratio of
A chemist needs to prepare a solution buffered at pH 4.30 using one of the following acids (and its sodium salt):
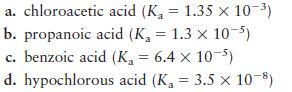
Calculate the ratio of [HA]/[A–] required for each system to yield a pH of 4.30. Which system will work best?
a. chloroacetic acid (K = 1.35 x 10-) b. propanoic acid (K = 1.3 x 10-5) c. benzoic acid (K = 6.4 x 10-5) d. hypochlorous acid (K = 3.5 10-8)
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
A pH of 430 corresponds to H 10430 antilog430 50 x 105 M Since K values rather tha... View full answer

Get step-by-step solutions from verified subject matter experts


