(a) Generally, the first ionization energies of elements in the same period increase upon going to higher...
Question:
(a) Generally, the first ionization energies of elements in the same period increase upon going to higher atomic number. Why?
(b) Examine the data for the p-block elements given in Fig. 1F.9. Note any exceptions to the rule given in part (a). How are these exceptions explained?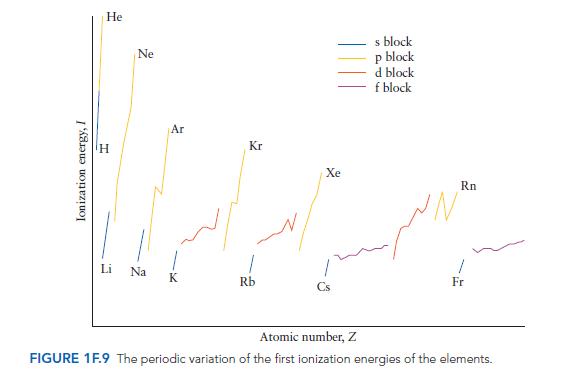
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





