A sample of hydrogen chloride gas, HCl, is being collected by bubbling it through liquid benzene. Assume
Question:
A sample of hydrogen chloride gas, HCl, is being collected by bubbling it through liquid benzene. Assume that the molecules pictured as spheres show a representative sample of the mixture of HCl and benzene vapor (⚫represents an HCl molecule and ⚪ a benzene molecule).
(a) Use the figure to determine the mole fractions of HCl and benzene vapor in the gas inside the container.
(b) What are the partial pressures of HCl and benzene in the container when the total pressure inside the container is 0.80 atm?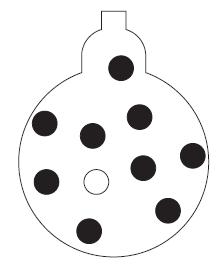
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





