A solution was prepared by dissolving 18.00 g of glucose in 150.0 g of water. The resulting
Question:
A solution was prepared by dissolving 18.00 g of glucose in 150.0 g of water. The resulting solution was found to have a boiling point of 100.34°C at 1 atm. Calculate the molar mass of glucose. Glucose is a molecular solid that is present as individual molecules in solution.
Transcribed Image Text:
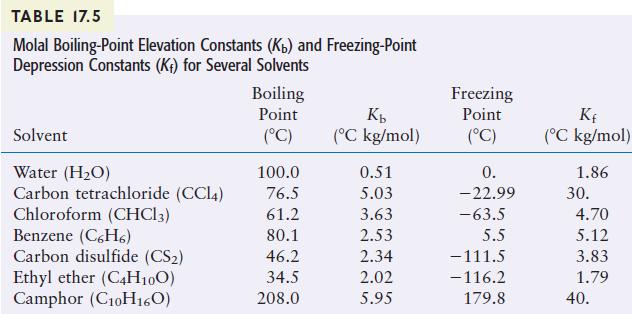
TABLE 17.5 Molal Boiling-Point Elevation Constants (Kb) and Freezing-Point Depression Constants (K) for Several Solvents Solvent Water (HO) Carbon tetrachloride (CC14) Chloroform (CHC13) Benzene (C6H6) Carbon disulfide (CS) Ethyl ether (C4H10O) Camphor (C10H160) Boiling Point (C) 100.0 76.5 61.2 80.1 46.2 34.5 208.0 Kb (C kg/mol) 0.51 5.03 3.63 2.53 2.34 2.02 5.95 Freezing Point (C) 0. -22.99 -63.5 5.5 -111.5 -116.2 179.8 Kf (C kg/mol) 1.86 30. 4.70 5.12 3.83 1.79 40.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
We make use of the equation AT Kumsolute AT 10034C 10000C 034...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solid is generated by revolving about the x-axis the region in the first quadrant bounded by the coordinate axes, linex = and graph of y = 1+ sin x. Which of the following integrals below will...
-
A solution was prepared by dissolving 0.834 g of sulfur, S8, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution.
-
A solution was prepared by dissolving 5.76 g of KCl MgCl2 6H2O (277.85 g/mol) in sufficient water to give 2.000 L. Calculate (a) The molar analytical concentration of KCl MgCl2 in this solution....
-
What is the best way to describe automation? developing highly advanced robots that can mimic higher-levelhuman thinking making human workers fully reliant on technology to performtheir job...
-
The table gives the population of the United States, in millions, for the years 19002000. (a) Use the exponential model and the census figures for 1900 and 1910 to predict the population in 2000....
-
The odds against Ishaq getting hired for a job are 3 : 8. Determine the probability (a) Ishaq gets hired. (b) Ishaq does not get hired.
-
Alternatives 1, 2, and 3 have lives of 3, 4, and 6 years, respectively. Their net cash flow (NCF) and salvage value (SV) profiles are as follows: Additional explanation is necessary: The NCF profile...
-
Elizabeth Soltis owns and operates Aunt Ibby's Styling Salon. A year-end work sheet is provided on the next page. Using this information, prepare adjusting entries, financial statements, and closing...
-
What output will be produced by the following code segment? int divide (double a, double b}{ } return (a/b); int main(){ double x=1.0, y = 4.0; cout < < divide(x, y); return 0; }
-
What mass of ethylene glycol (C 2 H 6 O 2 ), the main component of antifreeze, must be added to 10.0 L of water to produce a solution for use in a cars radiator that freezes at 10.0F (23.3C)? Assume...
-
A solution was prepared by adding 20.0 g of urea to 125 g of water at 25C, a temperature at which pure water has a vapor pressure of 23.76 torr. The observed vapor pressure of the solution was found...
-
a. You identify a novel microbe in your laboratory and find that it possesses two types of nucleic acid. Explain why you immediately rule out the fact that this microbe is a virus. b. Describe the...
-
What is meant by the term resource leverage? How does an understanding of this term help a firm exploit new product or service opportunities?
-
What is meant by the term strategic asset? Provide examples of the strategic assets of three well-known firms.
-
What is an international new venture? Explain why it might be to the benefit of an entrepreneurial start-up to position itself as an international new venture from the outset.
-
Describe the two potential pitfalls of using a team to start a firm.
-
A potential candidate in the third borough conducted a poll to decide whether he should challenge the incumbent. From a previous poll, he knows that the current incumbent has the support of 45% of...
-
On April 1, 1998, Miromar Tool Company authorized the sale of $8,000,000 of 7% convertible bonds with interest payment dates of April 1 and October 1. The bonds were sold on July 1, 1998, and mature...
-
At the beginning of the year, Lam Ltd. had total assets of $800,000 and total liabilities of $500,000. Use this information to answer each of the following independent questions. (a) If Lam's total...
-
What is the total entropy change accompanying a process in which 40.0 kJ of energy is transferred as heat from a large reservoir at 800. K to one at 200. K?
-
A good way to become familiar with thermodynamic processes is to start with a very simple system and consider how various changes might affect it. Calculate the change in molar Gibbs free energy, G m...
-
The composition of a compound used to make polyvinyl chloride (PVC) is 38.4% C, 4.82% H, and 56.8% Cl by mass. It took 7.73 min for a given volume of the compound to effuse through a porous plug, but...
-
Please explain using the key terms "Inspiron" and "OptiPlex" 8. Consider how Dell manufactures an Inspiron product line for individual consumers, and an OptiPlex product line for business customers....
-
Jackson seeks your advice as he wishes to ensure that the contractual relationship between both parties are sound in law. 1.1 You are required to fully advise Jackson as to what constitutes a valid...
-
HammerTime makes High quality tools. The company controller wants to calculate the fixed and variable cost associated with the janitorial cost incurred in the production factory. Data for the past...

Study smarter with the SolutionInn App


