(a) The standard potential of the reduction of Ag 2 CrO 4 to Ag(s) and chromate ions...
Question:
(a) The standard potential of the reduction of Ag2CrO4 to Ag(s) and chromate ions is 10.446 V. Write the balanced halfreaction for the reduction of silver chromate.
(b) Using the data from part(a) and Appendix 2B, calculate the solubility product of Ag2CrO4(s).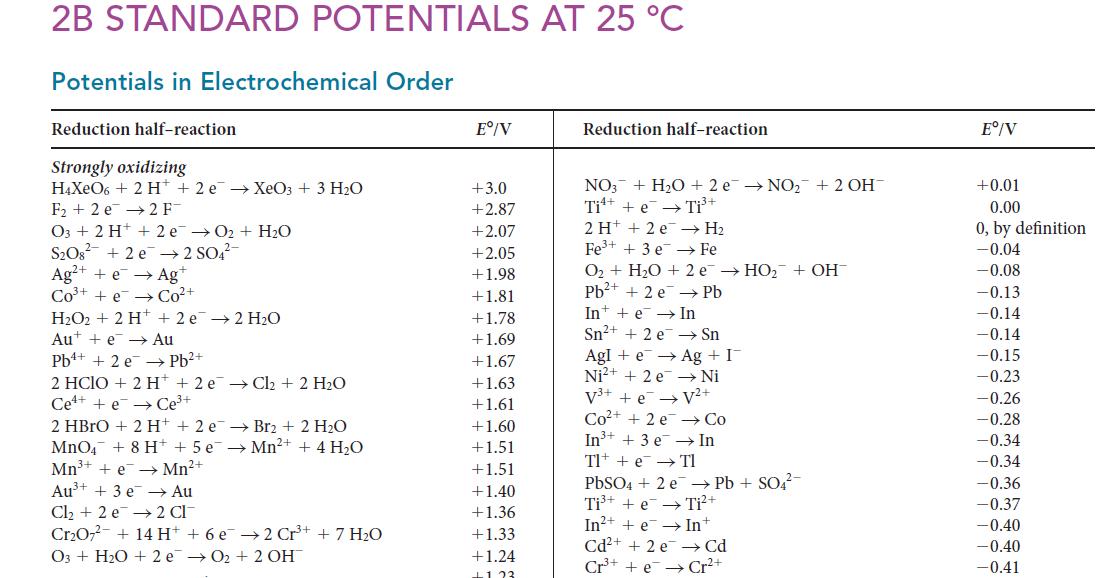
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 H₂O F₂2 e 2 F O3 + 2 H+ 2e →0₂ + H₂O S₂O8² +2 e 2 SO4²- Ag+ + e → Agt CO³+ +e Co²+ H₂O2 + 2 H+ + 2e → 2H₂O Aue Au Pb²+ Pb+ + 2 e 2 HClO + 2 H +2 e Cl₂ + 2 H₂O Cee Ce³+ 2 HBrO + 2 H+2 e → Br2₂ + 2 H₂O MnO4 + 8 H+ + 5 e¯ →Mn²+ + 4 H₂O Mn³+ + e→ Mn²+ Au³+ + 3e →→ Au Cl₂ +2 e 2 CI Cr₂O7² + 14 H+ + 6 e 03 + H₂O + 2 e 2 Cr³+ + 7 H₂O 0₂ + 2 OH™ Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +123 Reduction half-reaction NO3 + H₂O +2 e → NO₂ + 2 OH™ Ti + e →→ Ti³+ 2 H+ 2 e → H₂ Fe³+ + 3 e Fe O₂+ H₂O + 2 e → HO₂ + OH Pb²+ + 2e → Pb Int + e → In Sn²+ + 2 e Sn Agle → Ag + I Ni²+ + 2e → Ni V³+ + e → V²+ Co²+ +2e → Co In In³+ + 3 e Tl + e → TI PbSO4 + 2e Ti³+ + e In²+ + e→→ Int Cd²+ +2 e Cd Cr³+ + e→Cr²+ → Pb + SO4²- Ti²+ Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The balanced halfreaction for the reduction of silver chromate is Ag2CrO4s 8H 6e 2Ags CrO42 4H2O The ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The Manning Company has financial statements as shown next, which are representative of the company's historical average. The firm is expecting a 35 percent increase in sales next year, and...
-
The standard potential of the Zn2+/ Zn electrode is -0.76 V at 25e. The exchange current density for H+ discharge at platinum is 0.79 mA cm-2 Can zinc be plated on to platinum at that temperature?...
-
The exchange current density for a PtIFe3+,Fel+ electrode is 2.5 mA cm-I. The standard potential of the electrode is +0.77 V. Calculate the current flowing through an electrode of surface area 1.0...
-
9. Let x = (1.11... 111000...) 26, in which the fractional part has 26 1's followed by O's. For the Marc-32, determine x, x+, f(x), x-xx-x, xx, and lx-fl(x)/x.
-
Process further or sell, byproduct. (CMA, adapted) Newcastle Mining Company (NMC) mines coal, puts it through a one-step crushing process, and loads the bulk raw coal onto river barges for shipment...
-
You have been hired as the new controller for the Ralston Company. Shortly after joining the company in 2011, you discover the following errors related to the 2009 and 2010 financial statements: a....
-
List at least ten ambiguous words that should not be used in framing questions.
-
At the end of the current year, the accounts receivable account of Parkers Nursery Supplies has a debit balance of $350,000. Credit sales are $2,300,000. Record the end-of-period adjusting entry on...
-
According to the 8-step communication model, what should one do once the crisis has passed?
-
Calculate the molar concentrations of H 2 SO 3 , HSO 3 , SO 3 2 , H 3 O + , and OH present in 0.170 m Na 2 SO 3 (aq).
-
Suppose that each of the following pairs of redox couples is combined to form a galvanic cell that generates a current under standard conditions. Identify the oxidizing agent and the reducing agent,...
-
What kinds of changes did Gerstner makes in the companys HS&S operating groups? Since he became CEO of IBM in 2003, Sam Palmisano has worked hard to build a new global computer services company,...
-
What aspects of the time value of money must professional sport organizations and athletes consider when negotiating contracts?
-
How does a preference for liquidity influence an individual or organizations financial decisions?
-
A converging lens for which \(f=50 \mathrm{~mm}\) forms an image that is three times larger than the object. How far from the lens is the object?
-
What are some advantages and disadvantages of deferring salaries (both from the players and the teams perspectives)?
-
What mistake did the NBA make in its dealings with the owners of the Spirits of St. Louis?
-
Refer to data. You have allocated the costs from the Human Resources and General Administration departments to the P&R and FM departments. Suppose you have determined that the cost pool for the P&R...
-
After Theorem 1.5 we note that multiplying a row by 0 is not allowed because that could change a solution set. Give an example of a system with solution set S0 where after multiplying a row by 0 the...
-
Show that T = 1 + T ( In z/T) P , and that Pk = 1 P( In z/T) T.
-
A sample containing 42.1 g of Ar is enclosed in a container of volume 0.0885 L at 375 K. Calculate P using the ideal gas, van der Waals, and RedlichKwong equations of state. Based on your results,...
-
The experimental critical constants of CH 4 are found in Table 7.2. Use the values of Pc and T c to calculate V c . Assume that CH 4 behaves as a. An ideal gas b. A van der Waals gas c. A...
-
ATI Video Case Study: Delegation Overview This discussion requires you to access the ATI Testing website. To access ATI, use the ATI Testing link in the Canvas navigation menu or select this ATI...
-
As a COR, what can you do in support of the Program manager? Increase dollar limit of the contract Modify the contract Direct contractor employees or subcontractors Provide technical analysis on...
-
An analysis of current vision/mission and proposal for updated vision/mission of costco external Factor Evaluation Matrix and Porter's 5-Forces Analysis of costco. Internal Factor Evaluation Matrix...

Study smarter with the SolutionInn App


