A typical bathtub can hold 100. gallons of water. (a) Calculate the mass of natural gas that
Question:
A typical bathtub can hold 100. gallons of water.
(a) Calculate the mass of natural gas that would need to be burned to heat the water for a tub of this size from 65 °F to 108 °F. Assume that the natural gas is pure methane, CH4.
(b) What volume of natural gas does this correspond to at 25 °C and 1.00 atm? See Table 4D.1.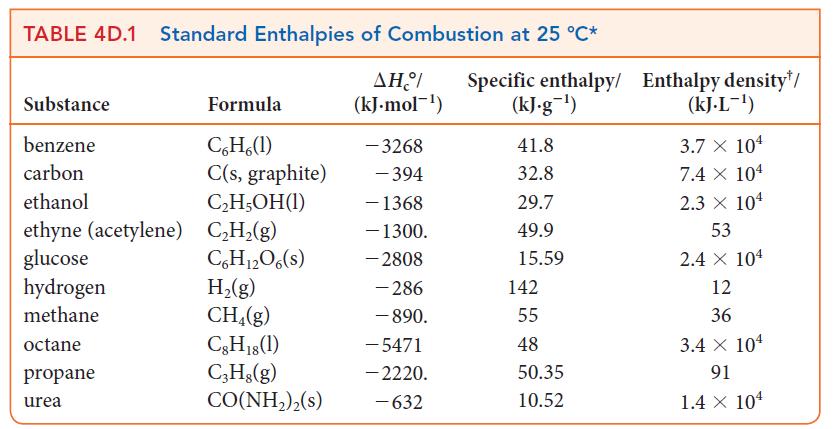
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





