Question: (a) Use the data in Appendix 2B to decide which of ozone and fluorine is the stronger oxidizing agent in water. (b) Does youranswer depend
(a) Use the data in Appendix 2B to decide which of ozone and fluorine is the stronger oxidizing agent in water.
(b) Does your answer depend on whether the reaction is carried out in acidic or basic solution?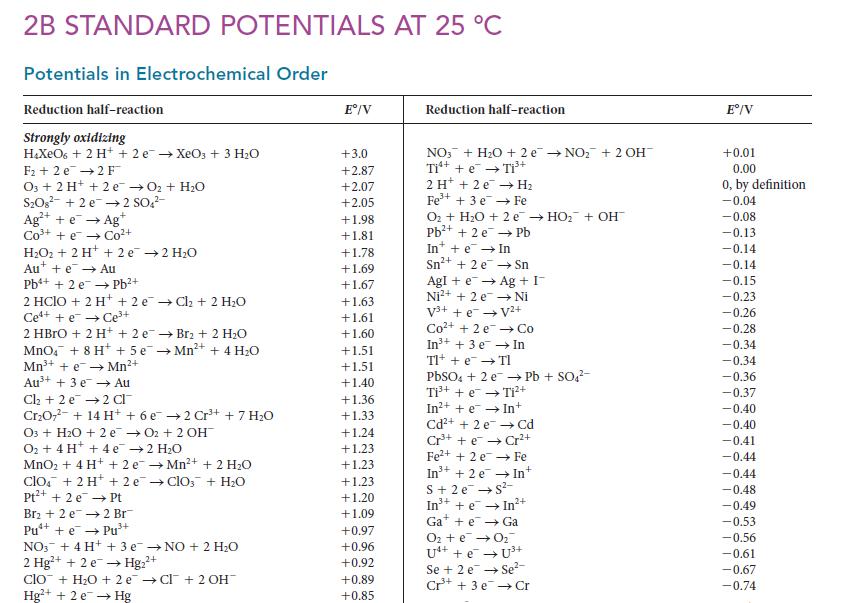
2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing HXeO6 + 2 H+ + 2 e XeO3 + 3 HO F2 e 2 F 03 + 2 H+ + 2 e O + HO SO8 +2e 2 SO4- Ag+ + e Agt C t cot HO+ 2 H+ + 2e 2 HO Aue Au Pb+ + 2 e Pb+ 2 HClO + 2 H+ +2e Ch + 2 HO Ce++eCe+ 2 HBrO + 2 H+ + 2 e MnO4 + 8 H+ + 5e Mn+ + eMn+ Au+ + 3 e Au Cl +2 e 2 Cl CrO7 + 14 H+ + 6 e 03 + HO + 2 e O + 4 H+ + 4e MnO + 4H+ + 2e clo + 2H+ + 2e Pt+ + 2 e Pt Br + 2 e2 Br Pu+ + ePu+ 3+ Br2 + 2 HO Mn+ + 4 H0 2 Cr+ + 7 HO 02 + 2 OH 2 HO Mn+ + 2 HO ClO3 + HO NO3 + 4 H+ + 3 eNO + 2 HO 2+ 2 Hg+ + 2e Hg+ clo + HO +2e Cl + 2 OH Hg+ + 2 e Hg E/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 +1.23 +1.23 +1.20 +1.09 +0.97 +0.96 +0.92 +0.89 +0.85 Reduction half-reaction NO3 + HO + 2e NO+ 2 OH Ti+ + e Ti+ 2 H + 2e H Fe+ + 3 e Fe O + HO + 2 e HO + OH Pb+ + 2e Pb In+ + e In Sn+ + 2 e Sn Ag + I Ni Agi + e Ni+ + 2 e V+ + e V+ Co+ +2 e In+ + 3 e Tl+ + e TI Co In PbSO4 + 2 e Ti+ + e Ti+ In+ + e In+ Cd+ + 2e Cd Cr+ +eCr+ Fe+ + 2e Fe In +2 e S+ 2e S- Pb + SO4- + In Gae Ga O + e 0 U4+ + e Se+ 2 e Cr+ +eIn2+ Se- + 3e Cr E/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44 -0.44 -0.48 -0.49 -0.53 -0.56 -0.61 -0.67 -0.74
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
The data in Appendix 2B shows the standard potentials of various halfreactionsThe standard potential ... View full answer

Get step-by-step solutions from verified subject matter experts


