Question: At a given temperature, K = 1.3 10 -2 for the reaction N(g) + 3H(g)2NH(g) Calculate values of K for the following reactions at
At a given temperature, K = 1.3 × 10-2 for the reaction
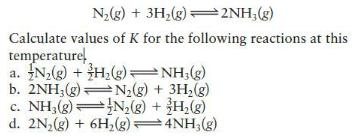
N(g) + 3H(g)2NH(g) Calculate values of K for the following reactions at this temperature a. N(g) + H(g) NH3(g) b. 2NH(g) c. NH3(g) d. 2N(g) + 6H(g) 4NH3(g) N(g) + 3H(g) N(g) + 3H(g)
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
Values of equilibrium constant at given temperature for the following reactions are 011 769 88 and 1... View full answer

Get step-by-step solutions from verified subject matter experts


