Boyle continued to add mercury to the apparatus in Exercise 3B.1 until the height of the trapped
Question:
Boyle continued to add mercury to the apparatus in Exercise 3B.1 until the height of the trapped air had been reduced to 6.85 in.. Assuming that atmospheric pressure had not changed, what was the pressure of the trapped air at that point (in inHg)?
Exercise 3B.1
Robert Boyle measured pressure in inches of mercury (inHg). On a day when the atmospheric pressure was 29.85 inHg, he trapped some air in the tip of a J-tube (1) and measured the difference in height of the mercury in the two arms of the tube (h). When h 5 12.0 in., the height of the gas in the tip of the tube was 32.0 in.. Boyle then added additional mercury and the level rose in both arms of the tube so that h 5 30.0 in. (2).
(a) What was the height of the air space (in inches) in the tip of the tube in (2)?
(b) What was the pressure of the gas in the tube in (1) and in (2) in inches of mercury?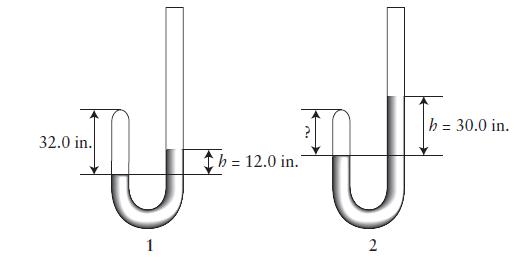
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





