Calculate the heat evolved or absorbed when 10.0 g of (a) NaCl; (b) NaI; (c) AlCl 3
Question:
Calculate the heat evolved or absorbed when 10.0 g of
(a) NaCl;
(b) NaI;
(c) AlCl3;
(d) NH4NO3 is dissolved in 100. g of water. Assume that the enthalpies of solution in Table 5D.3 are applicable and that the specific heat capacity of the solution is 4.18 J · K–1 · g–1.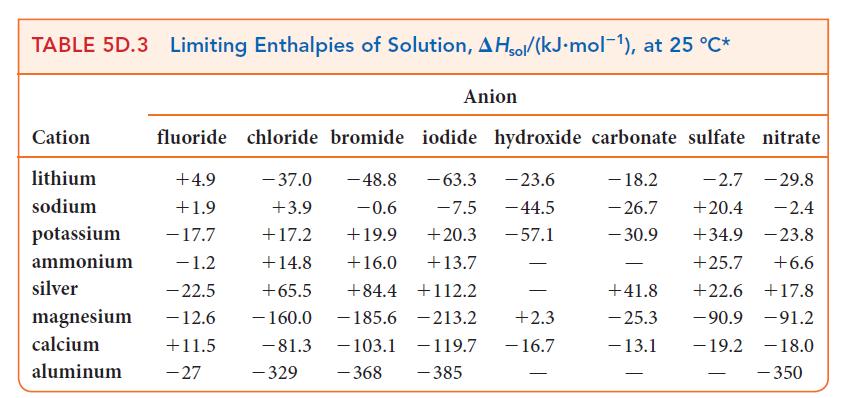
Transcribed Image Text:
TABLE 5D.3 Limiting Enthalpies of Solution, AHsol/(kJ.mol-¹), at 25 °C* Cation lithium sodium potassium ammonium silver magnesium calcium aluminum fluoride chloride bromide iodide hydroxide carbonate sulfate nitrate +4.9 - 37.0 -48.8 - 63.3 -23.6 - 18.2 -2.7 - 29.8 +1.9 +3.9 -0.6 -7.5 - 44.5 - 26.7 +20.4 -2.4 - 17.7 +17.2 +19.9 +20.3 -57.1 - 30.9 +34.9 -23.8 - 1.2 +14.8 +16.0 +13.7 +25.7 +6.6 -22.5 +65.5 +84.4 +112.2 +22.6 +17.8 - 12.6 - 160.0 -185.6 213.2 -90.9 -91.2 +11.5 -19.2 -103.1 - 119.7 -368 -385 -27 - 81.3 Anion -329 - +2.3 - 16.7 - +41.8 - 25.3 - 13.1 -18.0 - 350
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a 067 k...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In Problems 1922, use the method of Example 5 to find the constants A, B, and C in the indicated partial-fraction decompositions. 2 x(x - 1) X + B x-1 + C x + 1
-
In Exercises determine whether the statement is true or false. If it is false, explain why or give an example that shows it is false. lim x->0 x + ] x + x + 1 X = lim x-0 2x + 1 1 1
-
In Exercises 27 through 32, find the expected value for the random variable with the density function given in the indicated problem. a. P(1 X b. P(1 X 2)c. P(X 2) 3 f(x) = {x 0 if x 1 if x < 1
-
In Problems 1318, express the graph shown in blue using interval notation. Also express each as an inequality involving x. -1 0 1 2 3
-
A parallel combination of an 8- resistor and an unknown resistor R is connected in series with a 16- resistor and a battery. This circuit is then disassembled and the three resistors are then...
-
What are the three steps typically used to forecast sales for early-stage ventures?
-
Use $\mathrm{R}$ to calculate the three-way interaction effects from the experiment in Example 6.2 . Interpret these interaction terms. Data from Example 6.2 Example 6.2 (Silk Study). Silk fibroin is...
-
Grizzly Community Hospital in central Wyoming provides health care services to families living within a 200-mile radius. The hospital is extremely well equipped for a relatively small, community...
-
MyBnB started a home rental company on January 1. As of November 30, MyBnB reported the following balances. The company does not yet have a balance in Retained Earnings because this is its first year...
-
You are the executive assistant to the director of sales at B-Trendz, Inc., a trendy retail store that has locations in only ten states. The company is considering branching into the online retail...
-
(a) Calculate the reaction Gibbs free energy of N 2 (g)+ 3 H 2 (g) 2 NH 3 (g) when the partial pressures of N 2 , H 2 , and NH 3 are 4.2 bar, 1.8 bar, and 21 bar, respectively, and the temperature...
-
Colligative properties can be sources of insight into not only the properties of solutions, but also the properties of the solute. For example, acetic acid, CH 3 COOH, behaves differently in two...
-
A flashlight beam is reflected off a mirror lying flat on the ground. Use the information given below to find m2. 2
-
How is the three-month contract on SOFR settled?
-
What overnight rates are expected to replace LIBOR?
-
What is the purpose of applying a convexity adjustment to the rates obtained from futures quotes?
-
It is May 5, 2021. The quoted price of a government bond with a 12% coupon that matures on July 27, 2034, is 110-17. What is the cash price?
-
What is the formula for the minimum variance hedge ratio when daily settlement is ignored?
-
Your company is looking at purchasing a loader at a cost of $125,000. The loader would have a useful life of seven years. At the end of the seventh year the salvage value is estimated to be $10,000....
-
Write a paper about the Working relationship in the organization- collaboration within and outside the organization
-
Show that the expression (U/V) T = T(P/T)V P can be written in the form 2 / av
-
A sample containing 2.50 mol of an ideal gas at 325 K is expanded from an initial volume of 10.5 L to a final volume of 60.0 L. Calculate G and A for this process for a. An isothermal reversible path...
-
Why does the liquidgas coexistence curve in a PT phase diagram end at the critical point?
-
1. Observe the following historical (ex-post) returns of HUL, SAIL and Nifty: Returns (HUL Returns (SAIL Returns Year Stock) Stock) (Nifty) 1 30.00 26.00 35.00 2 19.00 13.00 20.00 3 30.00 48.00 32.00...
-
Estimate the 5 - day 9 9 % VaR for the portfolio assuming that its annual volatility calculated on the basis of weekly returns from the most recent 1 - year time window is 3 1 . 7 5 % .
-
You are saving up for your child's braces. You know you need $5,000 in 3 years when all his adult teeth have grown in. If your bank offers 4% interest, how much must you deposit today? Write a...

Study smarter with the SolutionInn App


