Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell
Question:
Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell potential. Balance the chemical equations by using the smallest whole-number coefficients.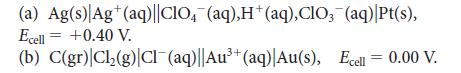
Transcribed Image Text:
(a) Ag(s) Ag+ (aq)||CIO4(aq),H+ (aq), CIO, (aq) |Pt(s), Ecell = +0.40 V. (b) C(gr) Cl₂(g) Cl¯(aq)||Au³+ (aq)| Au(s), Ecell = 0.00 V.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Ags Agaq ClO4aq Haq ClO3aq Pts Ecell 040 V Halfreactions Cathode Agaq e Ags E 0799 V Anode 6ClO3aq 6...View the full answer

Answered By

Ruksana K S
I have 2 year of experience in this field. I am engaged with reading, writing and my students as a child. Learning and teaching is part of my life.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell potential. Balance the chemical equations by using the smallest whole-number coefficients....
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
An electrochemical cell is set up using the following unbalanced reaction: Ma+(aq) + N(s) N2+(aq) + M(s) The standard reduction potentials are Ma+ + ae M o = 10.400 V N2+ + 2e- N o = 10.240 V The...
-
Mookie The Beagle Concierge Trial Balance As of January 31,2023 is given 1001 Checking 1010 Money Market 1100 Accounts Receivable (A/R) 1210 Prepaid Expenses:Supplies 1220 Prepaid Expenses:Insurance...
-
Partial productivity measurement Guble Company manufactures wallets from fabric. In 2008, Guble made 2,500,000 wallets using 1,875,000 yards of fabric. In 2009, Guble plans to make 2,650,000 wallets...
-
The long-term liability section of Eastern Post Corporation's balance sheet as of December 31, 2010, included 10% bonds having a face amount of $40 million and a remaining premium of $6 million. On...
-
Comment on the following field situations and make recommendations for corrective action. a. One of the interviewers has an excessive rate of refusals in in-home personal interviewing. b. In a CATI...
-
Healthy & Trim Co. offers personal weight reduction consulting services to individuals. After all the accounts have been closed on November 30, 2008, the end of the current fiscal year, the balances...
-
The grain stored inside a cylindrical silo is transferred to a transport container in the shape of a triangular prism. The container's triangular base has side lengths 5.8m, 5.8m, and 8.1m and height...
-
The pH of 0.50 m HBrO(aq) is 4.50. Calculate the change in pH when 5.10 g of sodium hypobromite is added to 100. mL of the solution. Ignore any change in volume.
-
A large volume of 0.250 m H 2 S(aq) is treated with a strong base to adjust the pH to 9.35. Assume that the addition of the base, a solid, does not significantly affect the volume of the solution....
-
Identify the place value of each underlined digit 513.8 9 9731
-
How would the following events (reported this year) affect your forecasts of a firms future profit or loss? An asset write-down. A merger or acquisition. The sale of a major division. The...
-
Consider two 5-year bonds; one has a $9 %$ coupon and sells for 101.00 ; the other has a $7 %$ coupon and sells for 93.20 . Find the price of a 5-year zero-coupon bond.
-
If the spot rates for 1 and 2 years are $s_{1}=6.3 %$ and $s_{2}=6.9 %$, what is the forward rate $f_{1,2}$ ?
-
Find the convexity of a zero-coupon bond maturing at time $T$ under continuous compounding (that is, when $m ightarrow \infty$ ).
-
Sports Research International hired Demarcus Jones, an African American, for the position of social media coordinator. Revise the following sentences to reduce bias (e.g., gender, racial, ethnic,...
-
For almost a year, Traki Company has been changing its manufacturing process from a traditional to a JIT approach. Management has asked for employees assistance in the transition and has offered...
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
If the total pressure is increased at constant T, how will the relative amounts of H 2 (g) and HCl(g) change? H 2 (g) + Cl 2 (g) 2HCl(g) at equilibrium. Assume ideal gas behavior.
-
Propose an efficient synthesis for each of the following transformations: a. b. c. d. Br
-
When methyl benzoate bears a substituent at the para position, the rate of hydrolysis of the ester moiety depends on the nature of the substituent at the para position. Apparently, a methoxy...
-
State of Economy You consider investing in two stocks, stock A and stock B. You have the following information Probability of State Return for stock A Return for stock B Economic 60% -20% 20%...
-
Estimate its beta and Jensen's alpha before, during, and after the recent COVID-19 financial crisis. Use the following dates for the three periods. Coca Cola stock price as of today $57.26. a. Before...
-
1. 2. Calculating Payback [L02] What is the payback period for the following set of cash flows? + Year Cash Flow 0 -$8,300 1 $2,100 2 $3,000 3 $2,300 4 $1,700 Calculating IRR [L05] A firm evaluates...

Study smarter with the SolutionInn App


