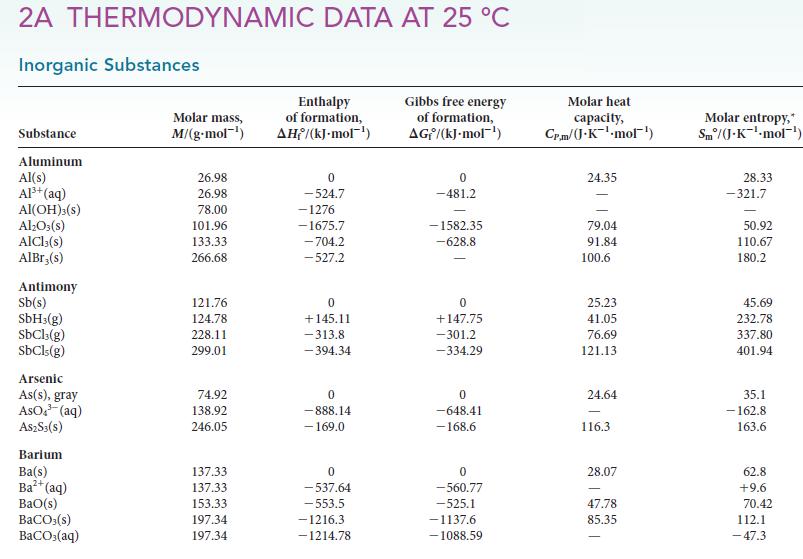
Calculate the standard reaction entropy, enthalpy, and Gibbs free energy for each of the following reactions from
Question:
Calculate the standard reaction entropy, enthalpy, and Gibbs free energy for each of the following reactions from data in Appendix 2A:
(a) The production of “synthesis gas,” a low-grade industrial fuel:![]()
(b) The thermal decomposition of ammonium nitrate:![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





