Consider a 0.60-M solution of HC 3 H 5 O 3 , lactic acid (Ka = 1.4
Question:
Consider a 0.60-M solution of HC3H5O3, lactic acid (Ka = 1.4 × 1024).
a. Which of the following are major species in the solution?
i. HC3H5O3
ii. C3H5O3
iii. H+
iv. H2O
v. OH-
b. Complete the following ICE table in terms of x, the amount (mol/L) of lactic acid that dissociates to reach equilibrium.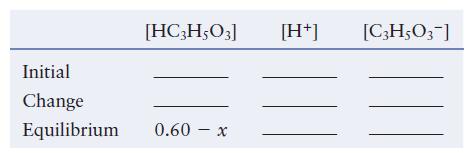
c. What is the equilibrium concentration for C3H5O3-?
d. Calculate the pH of the solution.
Transcribed Image Text:
Initial Change Equilibrium [HC3H5O3] 0.60 x - [H+] [C3H5O3-]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a The major species from above in...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of- the following are electron-deficient compounds? Explain. (a) (b) CH3 CH H C CHy H3C CH,
-
Which of the following are Section 1231 assets? Explain. Assume all the items have been held long-term. a. Machinery used in the business b. Personal home c. Factory building d. Land held as an...
-
Which of the following are private goods and might, therefore, be provided in socially optimal amounts by private profit-maximizers? Which are public goods and should, therefore, be provided by the...
-
You are the CEO of Green Paper Inc., a producer of high-end printing paper with an emphasis on environmentally friendly "green" production methods. One of your employees has proposed a significant...
-
Prove that the quantile function F1 of a general random variable X has the following three properties that are analogous to properties of the c.d.f.: a. F1 is a nondecreasing function of p for 0 < p...
-
When would the customer be willing to pay a premium price for a product or service? What pricing strategy would be appropriate under these circumstances?
-
Water is circulated from a large tank, through a filter, and back to the tank as shown in Fig. P8.96. The power added to the water by the pump is \(200 \mathrm{ft} \cdot \mathrm{lb} / \mathrm{s}\)....
-
Wedona Energy Consultants prepares adjusting entries monthly. Based on an analysis of the unadjusted trial balance at January 31, 2014, the following information was available for the preparation of...
-
How much work ( in joules) is done in lifting a 45.57 newton box from the floor to a table that is 0.73 meters above the floor? Round your final answer to two decimal places. A ball is thrown...
-
On August 27 of the current year, Bailey Corporation exchanged $25,000 of 4% interest-bearing bonds for 100 shares of its common stock worth $300 per share. If your answer amount is zero, enter "0"....
-
A 10.0-mL sample of an HCl solution has a pH of 2.000. What volume of water must be added to change the pH to 4.000?
-
A solution is prepared by adding 50.0 mL concentrated hydrochloric acid and 20.0 mL concentrated nitric acid to 300 mL water. More water is added until the final volume is 1.00 L. Calculate [H + ],...
-
A certain make of car comes equipped with an engine in one of four sizes (in liters): 2.8, 3.0, 3.3, or 3.8. Ten percent of customers order the 2.8 liter engine, 40% order the 3.0, 30% order the 3.3,...
-
Simplify by first writing the radicals with the same index. Then multiply. 35.54
-
This reflection activity is comprised of two sections collectively totaling a minimum of 500 words. Complete your reflections by responding to all prompts. Reflect on the following in a minimum of...
-
If a corporation issues 3,000 shares of $1.40 par value common stock for $18,000, the journal entry would include a credit to O A. Common Stock for $18,000. B. Paid-in Capital in Excess of Par-Common...
-
Dividing Partnership Net Income Steve Jack and Chelsy Boxer formed a partnership, dividing income as follows: 1. Annual salary allowance to Boxer of $191,400. 2. Interest of 6% on each partner's...
-
Assume that you are an accountant at XYZ Company. XYZ management has asked you to assist them with an issue. XYZ is considering the option to invest excess cash in short-term financial instruments....
-
Calculate the activation energy for vacancy formation in aluminum, given that the equilibrium number of vacancies at 500C (773 K) is 7.57 1023 m-3. The atomic weight and density (at 500C) for...
-
Choose two matrices A and B with dimension 2 x 2. Calculate det A, det B, and det (AB). Repeat this process until you are able to discover how these three determinants are related. Summarize your...
-
A compound contains only carbon, hydrogen, nitrogen, and oxygen. Combustion of 0.157 g of the compound produced 0.213 g of CO 2 and 0.0310 g of H 2 O. In another experiment, 0.103 g of the compound...
-
Maleic acid is an organic compound composed of 41.39% C, 3.47% H, and the rest oxygen. If 0.129 mole of maleic acid has a mass of 15.0 g, what are the empirical and molecular formulas of maleic acid?
-
Determine the molecular formula of a compound that contains 26.7% P, 12.1% N, and 61.2% Cl, and has a molar mass of 580 g/ mol.
-
1. Choose a key material from Table 02 above and document your choice of material and temper in your work. I used Tin Brass (Sy= 55000posi, Su=6000 ksi, E=16000, elongation: 6%) Hardness 76) 2....
-
Gold Nest Company of Guandong, China, makes birdcages for the South China market. The company sells its birdcages through an extensive network of street vendors who receive commissions on their...
-
1. It seems as though any problem involving the time value of money can be solved using any of the tables or equations. The key is to always make it easy on yourself. Solve it in a way that fits your...

Study smarter with the SolutionInn App


