Consider the ammonia synthesis reaction where G =33.3 kJ per mole of N 2 consumed at 25C.
Question:
Consider the ammonia synthesis reaction
![]()
where ΔG =–33.3 kJ per mole of N2 consumed at 25°C. For each of the
following mixtures of reactants and products at 25°C, predict the direction in
which the system will shift to reach equilibrium.
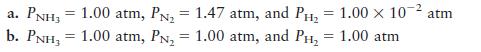
Transcribed Image Text:
N(g) + 3H(g) = 2NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a We can predict the direction of the shift to equilibrium by calculating the value of AG ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction 2NO2(g) N2O4(g) For each of the following mixtures of reactants and products at 25oC, predict the direction in which the reaction will shift to reach equilibrium. Use...
-
For the synthesis of ammonia at 500C, the equilibrium constant is 6.0 10 2 L 2 /mol 2 . Predict the direction in which the system will shift to reach equilibrium in each of the following cases. -3 a....
-
What is the output of the program if the input value is 202 input_value = 0 input_value = int(input('Input value: ')) if input_value < 10: print('Live') elif input_value < 20: print('Long') elif...
-
By applying modern technology to agriculture, the United States has become the most productive food-producing nation in the world. The secret to solving the world food security problem lies in...
-
A particle moves in a medium under the influence of a retarding force equal to mk(v3 + a2v), where k and a are constants. Show that for any value of the initial speed the particle will never move a...
-
Phenacetin was widely used as an analgesic before it was removed from the market in 1983 on suspicion of being a carcinogen. It was widely replaced with acetaminophen (Tylenol), which is very similar...
-
Mikes Powersports uses the LIFO inventory method. Mikes Powersports started August with 10 helmets that cost \($54\) each. On August 19, Mikes Powersports bought 15 helmets at \($56\) each. On August...
-
On July 1, 2014, Witherspoon Satellites issued $4,500,000, 9%, 10-year bonds at $4,219,600. This price resulted in an effective-interest rate of 10% on the bonds. Wither-spoon uses the...
-
With reference to the Schwartz article critiquing ethical codes: Do organizational policies about discrimination (on the basis of gender, race, or sexual identity) seem like useful ways to address...
-
The overall reaction for the corrosion (rusting) of iron by oxygen is Using the following data, calculate the equilibrium constant for this reaction at 25C. 4Fe(s) + 30(g) 2FeO3(s)
-
One method for synthesizing methanol (CH 3 OH) involves reacting gaseous carbon monoxide and hydrogen: Calculate G at 25C for this reaction, in which carbon monoxide gas at 5.0 atm and hydrogen gas...
-
The following questions relate to the use of analytical procedures in general planning. Select the best response. a. Analytical procedures are 1. Statistical tests of financial information designed...
-
Your client has $ 1 5 , 9 0 3 already in a retirement account, and she plans to add a $ 9 , 6 4 5 deposit one year from today, and increase that annual deposit by 2 % per year ending 3 5 years from...
-
Trial Balance When should this be done by? End of the month Who should complete this report? A) Explain how this figure is achieved B) What records are used? What does it represent? Cashbook...
-
The Blackstar Fund has assets with a market value of $12.6 million and liabilities of $800,000. What is the net asset value (NAV) if there are 200,000 shares outstanding?
-
Given an interest rate of 8 % , how much should you invest now in order to produce $ 3 , 0 0 0 at the end of the year?
-
(a) Represent this game in the extensive form, i.e. game tree.
-
Why do management accountants need to understand their companys strategy?
-
The graph of the sequence whose general term is an = n - 1 is which of the following? [8.1] A. B. TITTT 3-2-1 23.45 2.3.4
-
The molar concentration of HCl in hydrochloric acid is reduced to 12% of its initial value by dilution. What is the difference in the pH values of the two solutions?
-
Salts are often used to create solutions with specific pH values. Suppose you need to prepare a salt solution with a pH of about 4.5 and have available sodium dihydrogen phosphate, NaH 2 PO 4 , and...
-
Calculate the standard reaction Gibbs free energy for the following cell reactions: 4+ (a) 2 Ce (aq) + 3I (aq) 2 Ce(aq) + 13 (aq), Ecell +1.08 V (b) 6 Fe+(aq) +2 Cr+(aq) + 7 HO(1) 6 Fe+ (aq) + CrO2...
-
Let x=(-11,-15). Find a vector, n, in the opposite direction of x such that ||n|| = 7. Enter your answer in exact form. Provide your answer below:
-
CH-CH, reacts 1000 times faster than Ph-CH=CH, with Br-HOAc but only 3.8 times faster with pCI-CH,SCI in HOAC at 25C. Explain.
-
Divide the following and check by multiplication: 43)2,661 Quotient Remainder

Study smarter with the SolutionInn App


