Question: Consider the following data: b. Based on your answer to part a, which is the stronger oxidizing agent, Co 3+ or Co(en) 3 3+ ?
Consider the following data:
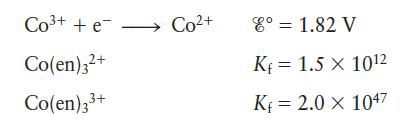
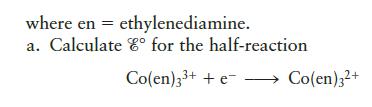
b. Based on your answer to part a, which is the stronger oxidizing agent, Co3+ or Co(en)33+?
c. Use the crystal field model to rationalize the result in part b.
Co+ + e- Co(en)3+ Co(en)3+ Co+ 8 = 1.82 V Kf = 1.5 X 102 K 2.0 X 1047
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Answer a The potential for the halfreaction Coen33e Coen32 can be ca... View full answer

Get step-by-step solutions from verified subject matter experts


