Question: Consider the process which is carried out at constant pressure. The total S for this process is known to be 75.0 J K21 mol21. For
Consider the process
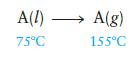
which is carried out at constant pressure. The total △S for this process is known to be 75.0 J K21 mol21. For A(l) and A(g), the Cp values are 75.0 J K21 mol21 and 29.0 J K21 mol21, respectively, and are not dependent on temperature. Calculate △H vaporization for A(l) at 1258C.
A(l) A(g) 75C 155C
Step by Step Solution
3.43 Rating (178 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


