Question: Consider the reaction between (mathrm{NO}(g)) and (mathrm{O}_{2}(g)) represented below. What is the balanced equation for this reaction and what is the limiting reactant? 0 NO
Consider the reaction between \(\mathrm{NO}(g)\) and \(\mathrm{O}_{2}(g)\) represented below.
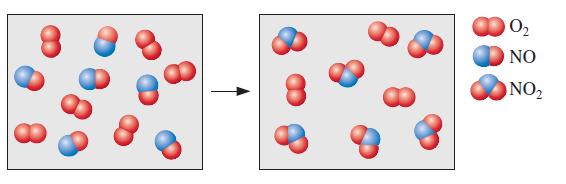
What is the balanced equation for this reaction and what is the limiting reactant?
0 NO NO
Step by Step Solution
★★★★★
3.45 Rating (161 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Based on the image it appears that we are dealing with a reaction where nitrogen monoxide NO reacts with oxygen O2 to form nitrogen dioxide NO2 While ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


