Depending on the temperature, rubidium chloride can exist in either the rock-salt (Fig. 3H.25) or cesium-chloride structure
Question:
Depending on the temperature, rubidium chloride can exist in either the rock-salt (Fig. 3H.25) or cesium-chloride structure (Fig. 3H.30).
(a) What are the coordination numbers of the rubidium and chloride ions in each structure?
(b) In which of these structures does the rubidium ion occupy the larger “hole”?
FIGURE 3H.30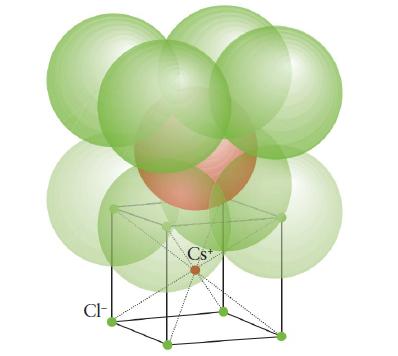
FIGURE 3H.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





