Dissociation of a diatomic molecule, X 2 (g) 2 X(g)occurs at 500 K. The equilibrium state of
Question:
Dissociation of a diatomic molecule, X2 (g) ⇌ 2 X(g) occurs at 500 K. The equilibrium state of the reaction is shown in 1 and the equilibrium state in the same container after a change has occurred is shown in 2. Which of the following changes will produce the composition shown?
(a) Increasing the temperature.
(b) Adding X atoms.
(c) Decreasing the volume.
(d) Adding a catalyst. Explain your selections.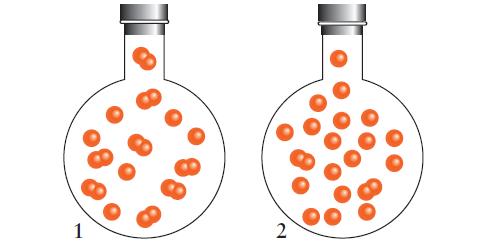
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





