Question: Does the information on alkali metals in Table 12.9 of the text confirm the general periodic trends in ionization energy and atomic radius? Explain. Table
Does the information on alkali metals in Table 12.9 of the text confirm the general periodic trends in ionization energy and atomic radius? Explain.
Table 12.9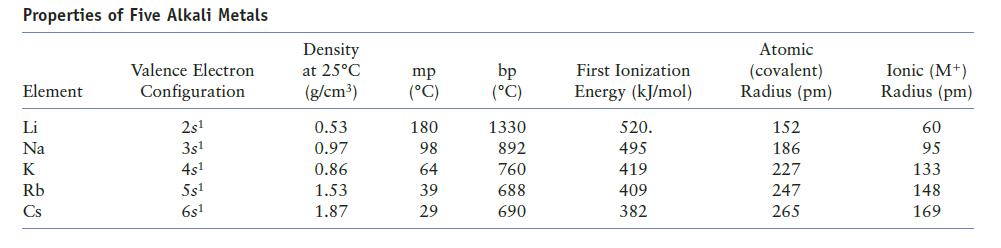
Properties of Five Alkali Metals Element Li Na K Rb Cs Valence Electron Configuration 2s 3s 4s 5s 6s1 Density at 25C (g/cm) 0.53 0.97 0.86 1.53 1.87 mp (C) 180 98 64 39 29 bp (C) 1330 892 760 688 690 First Ionization Energy (kJ/mol) 520. 495 419 409 382 Atomic (covalent) Radius (pm) 152 186 227 247 265 Ionic (M+) Radius (pm) 60 95 133 148 169
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
Answer The information on alkali metals in Table 129 does confirm the general ... View full answer

Get step-by-step solutions from verified subject matter experts


