Draw an orbital energy-level diagram (like those in Figs. 9D.3 and 9D.5) showing the configuration of d-electrons
Question:
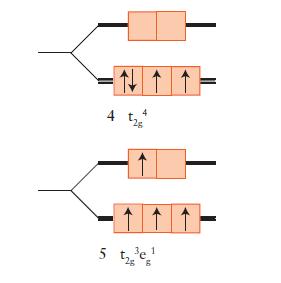
Draw an orbital energy-level diagram (like those in Figs. 9D.3 and 9D.5) showing the configuration of d-electrons on the metal ion in each of the following complexes:
(a) [Co(NH3)6]3+;
(b) [NiCl4]2– (tetrahedral);
(c) [Fe(OH2)6]3+;
(d) [Fe(CN)6]3–.
Predict the number of unpaired electrons for each complex.
FIGURE 9D.3
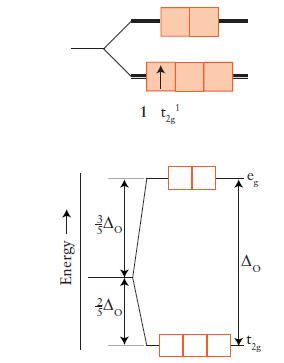
FIGURE 9D.5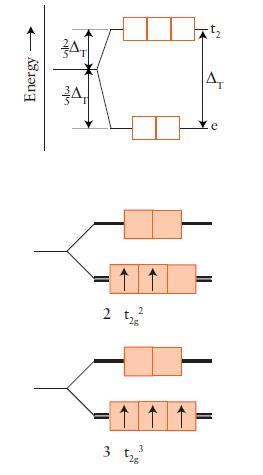
Transcribed Image Text:
دار Energy → داری 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a octahedral strongfield ligand 6 e no unpaired electrons 31...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Draw an orbital energy-level diagram (like those in Figs.9D.3 and 9D.5) showing the configuration of d-electrons on the metal ion in each of the following complexes: (a) [Zn(OH 2 ) 6 ] 2+ ; (b) [CoCl...
-
Draw a molecular orbital energy level diagram for each of the following species: He+2, HHe, He2+. Compare their relative stabilities in terms of bond orders. (Treat HHe as a diatomic molecule with...
-
You are working on a free-form Packet Tracer challenge activity as seen in Figure 1, you have been given the London Railways network.' The purpose of this EMA question is to build upon each of the...
-
Describes 7shifts' pioneering role in revolutionizing restaurant management through cloud-based solutions.
-
In the solution to the bounded buffer problem (Figure), consider the ordering of the first two P operations in the producer and the consumer. Suppose the order of the p(full) and the p(mutex)...
-
Shilstone Supply, Inc. manufactures a variety of pumps and valves that are distributed through several thousand plumbing supply houses, as well as 100 manufacturer's representatives. Due to lessthan...
-
For each of the following situations, calculate the \(t\)-statistic \((t)\) : a. \(\mathrm{X}^{-}=20.00 ; \mu=18 ; s \mathrm{X}^{-}=1.00\) b. \(X^{-}=20.00 ; \mu=13 ; s X^{-}=1.00\) c. \(X^{-}=12.00...
-
Was Jay Cohens conviction justified?
-
Bleakhouse Bakers produces breads and pastries. Bleakhouse is considering introducing a new breakfast item. They estimate materials costs at $4.00 per dozen and overhead costs of $2.02 per dozen....
-
New pharmaceuticals must be enantiomerically pure for use in human medicine. If you work for a large biotechnology company, you will need to be able to recognize chiral sites in complex molecules....
-
Use the information in Table 9C.1 to write the formula for each of the following coordination compounds: (a) Triamminediaquabromidocobalt(II) hydroxide (b) Dichloridobisethylenediaminecobalt(III)...
-
1. What is the Fed and what is the FOMC? 2. Who is the Feds chief executive, and what are the Feds main policy tools? 3. What is the monetary base? 4. Suppose that at the end of December 2009, the...
-
The following table shows an abbreviated income statement and balance sheet for Quick Burger Corporation for 2022. INCOME STATEMENT OF QUICK BURGER CORPORATION, 2022 (Figures in $ millions) Net sales...
-
Wight of the brass cube:66.92 PART A 1 2 3 5 6 7 8 9 10 N MAV+VAM y Length ruler (mm) 20 20.1 19.5 20.2 20.4 19.3 20.2 20.5 19.6 19.2 at al 199 |(al-d)| p accepted-p average Ap 179 178.9 179.5 178.8...
-
8. If D=A- B, calculate the uncertainty in SD. 8D = 0.02 0.14 0.20 a. b. c. 1
-
You wish to determine whether a chunk of platinum is made of pure platinum or of a particular platinum alloy. To aid you in this determination, your friends Ed and Ted have each independently...
-
5. Starting from the curl of E in Maxwell's equation, show that the new vector E' = E+ A/at can be expressed as the a gradient of scalar potential, in case of time dependent fields in vacuum. Write...
-
How is the food-purchasing system related to the food and beverage cost control system?
-
Provide an example of an aggressive accounting practice. Why is this practice aggressive?
-
Consider the energy-level diagrams depicted in the text. a. At what temperature will the probability of occupying the second-energy level be 0.15 for the states depicted in part (a) of the figure? b....
-
Consider the energy-level diagrams, modified from Problem P30.9 by the addition of another excited state with energy of 600. cm 1 . a. At what temperature will the probability of occupying the second...
-
Consider the following sets of populations for four equally spaced energy levels: a. Demonstrate that the sets have the same energy. b. Determine which of the sets is the most probable. c. For the...
-
a. A single person with earned income of $5,300 and no qualifying children. b. A single person with earned income of $23,600 and two qualifying children. c. A married couple filing jointly with...
-
u(x) Let u(x)=sin(x) and v(x) = x and f(x) v(x) u'(x) = v'(x) = u'v- uv' f' = v2
-
Tech Solutions is a consulting firm that uses a job - order costing system. Its direct materials consist of hardware and software that it purchases and installs on behalf of its clients. The firm s...

Study smarter with the SolutionInn App


