Glucose (C 6 H 12 O 6 ) and benzophenone (C 6 H 5 COC 6 H
Question:
Glucose (C6H12O6) and benzophenone (C6H5COC6H5) are examples of compounds that form molecular solids. The structures of glucose and benzophenone are given here.
(a) What types of forces hold these molecules in a molecular solid?
(b) Which of the two solids has the higher melting point?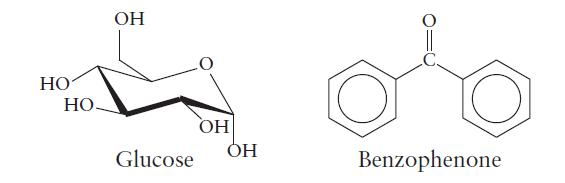
Transcribed Image Text:
HO НО- OH Glucose OH OH Benzophenone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a Glucose will be held in the solid by Lond...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Chloromethane (CH 3 Cl) and acetic acid (CH 3 COOH) form molecular solids. (a) What types of forces hold these molecules ina molecular solid? (b) Which of the two liquids has the higher freezing...
-
Classify each of the following materials as falling into one of the categories listed in Table 12.2. What particles make up these solids and what are the forces of attraction between particles? Give...
-
Rationalize why chalk (calcium carbonate) has a higher melting point than motor oil (large compounds made from carbon and hydrogen), which has a higher melting point than water, which engages in...
-
One popular activity that tourists participate in when they visit Alaska is panning for gold. A gift shop by the panning center sells blocks of clay. The packaging on the clay claims that one in five...
-
The primary purpose of managerial accounting is to provide information useful for management decisions. Many of the managerial accounting techniques that you learn in this course will be useful for...
-
Which of the following is xx + 1 dx? (a) (b) } (x + 1)5/ - (x + 1)/ + C
-
Describe the conditions under which non-monetary items designated in a foreign currency are subsequently remeasured under AASB 121/IAS 21?
-
Jay is single and works as a salesman. In December of the current year, he is selected as the company's outstanding salesperson. In recognition of this honor, he receives a $75,000 bonus, which puts...
-
1. Electric potential is given by V=6x-8xy-8y+6yz - 4z Then magnitude of electric force acting on 2C point charge placed on origin will be :- (1) 2N (2) 6N (3) 8N (3) 8N (4) 20 N 2. Figure shows...
-
The pressure of a sample of hydrogen fluoride is lower than expected and, as the temperature is increased, rises more quickly than the ideal gas law predicts. Suggest an explanation.
-
One reason that copper is so valuable is its high malleability, which is related to its structure. Suppose you have been asked to examine how different types of heat treatment affect the crystal...
-
Which of the following ratios is used to analyze liquidity? a. Earnings per share. b. Debt-to-assets. c. Current ratio. d. Both b and c.
-
Kenneth King inherited 100 shares of Corporation \(A B C\) stock from his father, who died on March 4, 2018. His father paid \$17 per share for the stock on May 3, 1998. The fair market value of the...
-
Salesforce.com earned the top spot on Fortunes list of the Best Companies to Work for in 2018, having been on the list for over 10 years. Use your university librarys resources to see what their...
-
Develop several other SKU priority rules. Discuss the advantages and disadvantages of each one.
-
The development of fugacity is stated to be inductive. Explain what you think is meant by that.
-
How would you estimate daily requirements in practice (i.e., how would you get the data for Exercise 6.6)?
-
The T-accounts for Equipment and the related Accumulated Depreciation for Stone Kitchen Equipment Company at the end of 2008 are as follows. Stone Kitchen Equipment Companys income statement reported...
-
Coastal Refining Company operates a refinery with a distillation capacity of 12,000 barrels per day. As a new member of Coastal's management team, you have been given the task of developing a...
-
A metal ion in a high-spin octahedral complex has two more unpaired electrons than the same ion does in a low-spin octahedral complex. Name some possible metal ions for which this would be true.
-
What is wrong with the following formulaname combinations? Give the correct names for each. a. [Cu(NH 3 ) 4 ]Cl 2 Coppermine chloride b. [Ni(en) 2 ]SO 4 bis(ethylenediamine)nickel(IV) sulfate c....
-
Which is more likely to be paramagnetic, Fe(CN) 6 4- or Fe(H 2 O) 6 2+ ? Explain.
-
HANDOUT 2-COST-VOLUME-PROFIT ANALYSIS THIS HANDOUT CONSISTS OF PARTS A AND B PART A Storbeck Sports sells decals that can be personalized with a player's name, a team name, and a jersey number for $5...
-
Given the following function, what are the asymptotic running times? Write it in asymptotic notation and justify why. (, O, ). def func1(n): x = 0: for i in range(1, n//2+1): for j in range(i, n-1...
-
ABC Co. purchased a used construction vehicle from another company that was liquidating its equipment. The purchase price was $150,000, and sales tax was 6%. The vehicle had to be transported to...

Study smarter with the SolutionInn App


