Question:
Hemoglobin contains one heme group per subunit. The heme group is an Fe2+ complex that is coordinated to the four N atoms in a porphyrin ligand in a square planar arrangement and to an N atom on the histidine residue (see Table 11E.3) on the subunit. The hemoglobin molecule transports O2 through the body by using a bond between O2 and the Fe2+ ion at the center of the heme group. The Fe2+ ion can be viewed as having octahedral coordination, with one position unoccupied and available for bonding to oxygen. (See Box 9C.1.)
(a) Draw the structure of the heme group with the sixth site not coordinated.
(b) The deoxygenated heme is a high-spin complex of the Fe2+ ion. When the oxygen molecule binds to the Fe2+ ion as the sixth ligand, the resulting octahedral complex is low spin. Predict the number of unpaired electrons in (i) deoxygenated and (ii) oxygenated heme.
(c) Other compounds can bond to the iron atom, displacing oxygen. Identify which of the following species cannot bond to the iron in a heme group and explain your reasoning: CO; Cl–; BF3; NO2–.
(d) Hemoglobin in both its oxygenated (HbO2) and deoxygenated (Hb) forms helps to maintain the pH of blood at an optimal level. Hemoglobin has several acidic protons, but the most important deprotonation is the first. At 25°C, for the deoxygenated form, HbH(aq) + H2O(l) ⇌ Hb–(aq) + H3O+(aq) and pKa1 = 6.62. For the oxygenated form, HbO2H(aq) + H2O(l) ⇌ HbO2–(aq)+ H3O+(aq) and pKa1 = 8.18. Calculate the percentage of each form of hemoglobin that is deprotonated at the pH of blood, 7.4.
(e) Use your answer to part (d) to determine how the oxygenation of hemoglobin affects the pH of blood. That is, will the pH increase, decrease, or stay the same as more hemoglobin molecules become oxygenated? Explain your reasoning.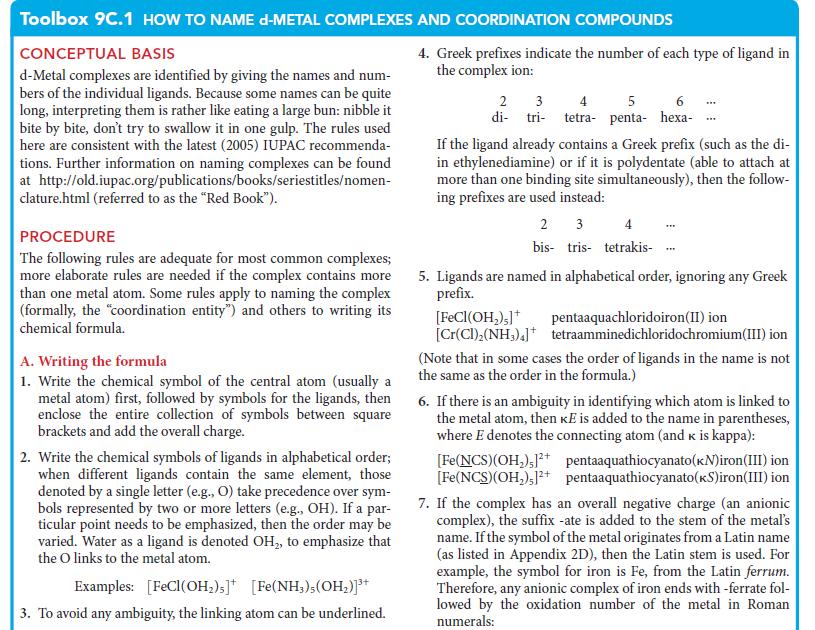
![Examples: [Fe(NCS) (OH), ]2+ [Fe(NCS) (OH), ]+ B. Naming the complex 1. Name the ligands first, and then the](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/1/1/0/5806592a9f48feaf1704110579446.jpg)
Transcribed Image Text:
Toolbox 9C.1 HOW TO NAME d-METAL COMPLEXES AND COORDINATION COMPOUNDS CONCEPTUAL BASIS d-Metal complexes are identified by giving the names and num- bers of the individual ligands. Because some names can be quite long, interpreting them is rather like eating a large bun: nibble it bite by bite, don't try to swallow it in one gulp. The rules used here are consistent with the latest (2005) IUPAC recommenda- tions. Further information on naming complexes can be found at http://old.iupac.org/publications/books/seriestitles/nomen- clature.html (referred to as the "Red Book"). PROCEDURE The following rules are adequate for most common complexes; more elaborate rules are needed if the complex contains more than one metal atom. Some rules apply to naming the complex (formally, the coordination entity") and others to writing its chemical formula. A. Writing the formula 1. Write the chemical symbol of the central atom (usually a metal atom) first, followed by symbols for the ligands, then enclose the entire collection of symbols between square brackets and add the overall charge. 2. Write the chemical symbols of ligands in alphabetical order; when different ligands contain the same element, those denoted by a single letter (e.g., O) take precedence over sym- bols represented by two or more letters (e.g., OH). If a par- ticular point needs to be emphasized, then the order may be varied. Water as a ligand is denoted OH, to emphasize that the O links to the metal atom. Examples: [FeCl(OH)s] [Fe(NH3), (OH)]+ 3. To avoid any ambiguity, the linking atom can be underlined. 4. Greek prefixes indicate the number of each type of ligand in the complex ion: 2 3 di-tri- 4 5 6 tetra- penta- hexa- If the ligand already contains a Greek prefix (such as the di- in ethylenediamine) or if it is polydentate (able to attach at more than one binding site simultaneously), then the follow- ing prefixes are used instead: [FeCl(OH);]* [Cr(CI), (NH3)4] 2 3 bis tris tetrakis- *** THE 5. Ligands are named in alphabetical order, ignoring any Greek prefix. pentaaquachloridoiron (II) ion tetraamminedichloridochromium(III) ion (Note that in some cases the order of ligands in the name is not the same as the order in the formula.) 6. If there is an ambiguity in identifying which atom is linked to the metal atom, then Ke is added to the name in parentheses, where E denotes the connecting atom (and k is kappa): [Fe(NCS)(OH),1+ pentaaquathiocyanato(kN)iron (III) ion [Fe(NCS)(OH), ]2+ pentaaquathiocyanato (kS)iron(III) ion 7. If the complex has an overall negative charge (an anionic complex), the suffix -ate is added to the stem of the metal's name. If the symbol of the metal originates from a Latin name (as listed in Appendix 2D), then the Latin stem is used. For example, the symbol for iron is Fe, from the Latin ferrum. Therefore, any anionic complex of iron ends with -ferrate fol- lowed by the oxidation number of the metal in Roman numerals:

![Examples: [Fe(NCS) (OH), ]2+ [Fe(NCS) (OH), ]+ B. Naming the complex 1. Name the ligands first, and then the](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/1/1/0/5806592a9f48feaf1704110579446.jpg)






