How close are the Mulliken and Pauling electronegativity scales? (a) Use Eq. 2 in Topic 2D to
Question:
How close are the Mulliken and Pauling electronegativity scales?
(a) Use Eq. 2 in Topic 2D to calculate the Mulliken electronegativities of C, N, O, and F. Use the values in kilojoules per mole from Figs. 1F.8 and 1F.12 and divide each value by 230 kJ · mol-1 for this comparison. The Pauling values are those in Fig. 2D.2.
(b) Plot both sets of electronegativities against atomic number on the same graph.
(c) Which scale depends more consistently on position in the periodic table?![]()
Fig. 2D.2
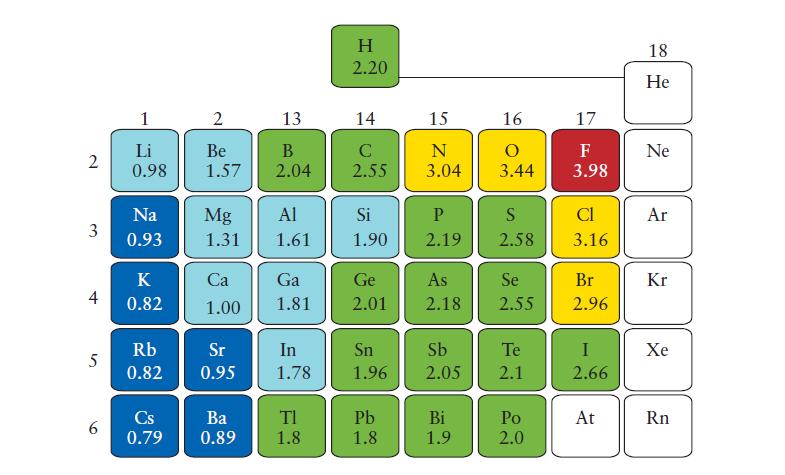
Fig. 1F.12
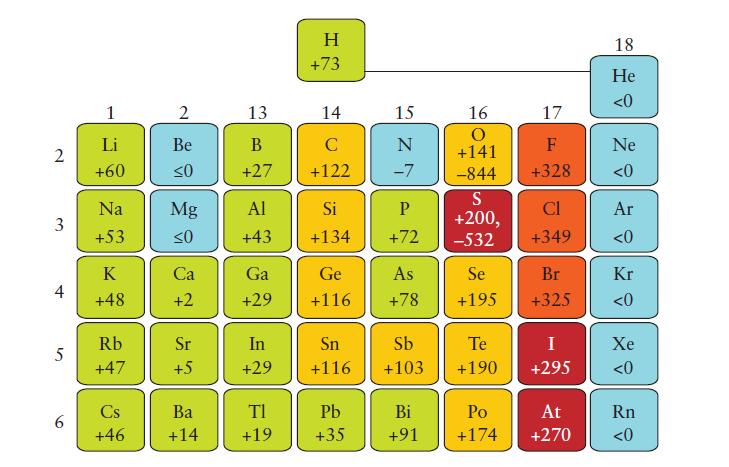
Fig. 1F.8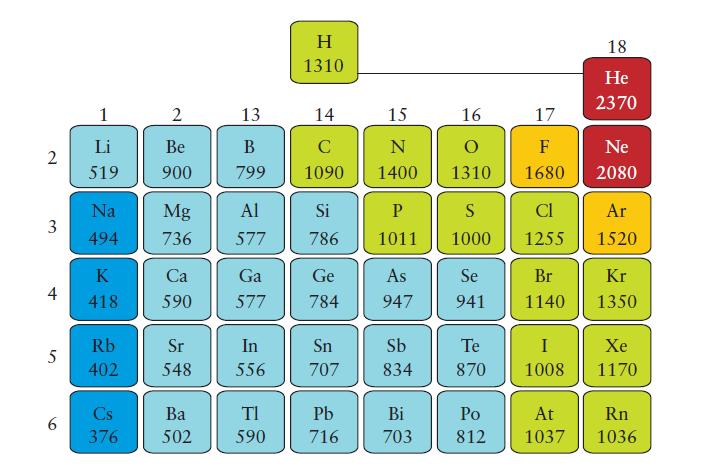
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





