Hydrogen peroxide, H 2 O 2 , reacts with sulfur trioxide to form peroxomonosulfuric acid, H 2
Question:
Hydrogen peroxide, H2O2, reacts with sulfur trioxide to form peroxomonosulfuric acid, H2SO5, in a Lewis acid–base reaction.
(a) Write the chemical equation for the reaction.
(b) Draw the Lewis structures of the reactants and product (in the product, one —OH group in sulfuric acid is replaced by an —OOH group).
(c) Identify the Lewis acid and Lewis base.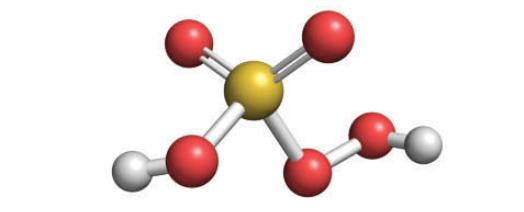
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





