Identify the reactions with K > 1 among the following reactions and, for each such reaction, write
Question:
Identify the reactions with K > 1 among the following reactions and, for each such reaction, write balanced reduction and oxidation half-reactions. For those reactions, show that K > 1 by calculating the standard Gibbs free energy of the reaction. Use the smallest whole-number coefficients to balance the equations.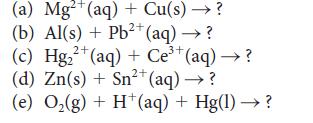
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





