In hot, dry climates an inexpensive alternative to air conditioning is the swamp cooler. In this device,
Question:
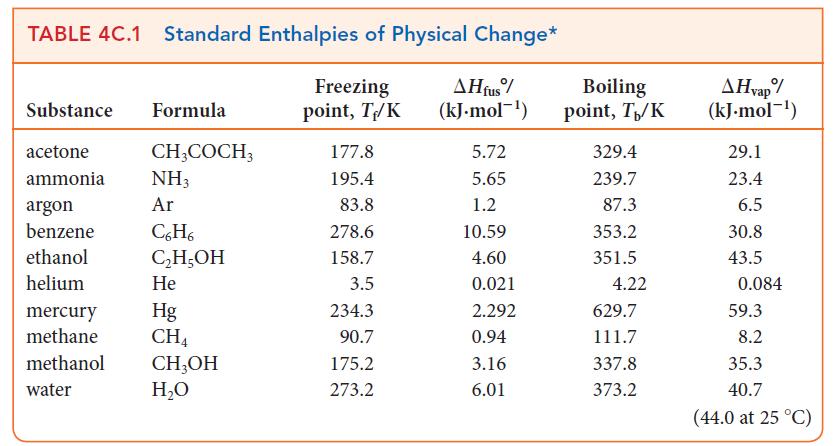
In hot, dry climates an inexpensive alternative to air conditioning is the swamp cooler. In this device, water continuously wets porous pads through which fans blow the hot air. The air is cooled as the water evaporates. Use the information in Tables 4A.2 and 4C.1 to determine how much water must be evaporated to cool the air in a room of dimensions 4.0 m * 5.0 m * 3.0 m by 20. °C. Assume that the enthalpy of vaporization of water is the same as it is at 25 °C.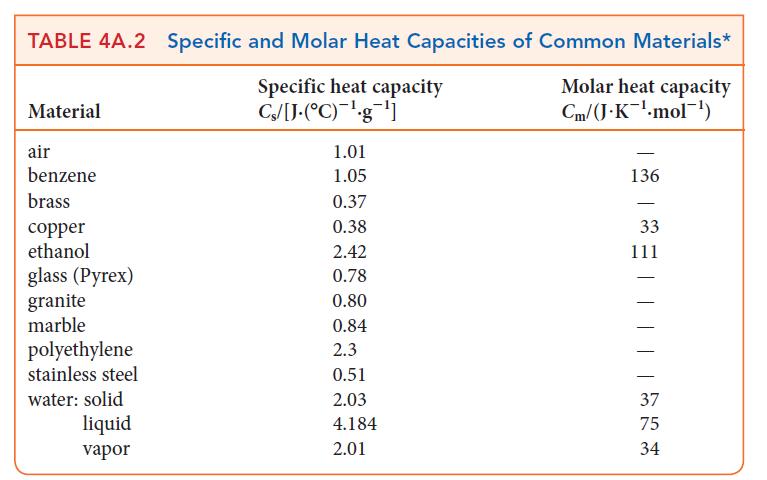
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





