Investigate whether the replacement of a carboncarbon double bond by single bonds is energetically favored by using
Question:
Investigate whether the replacement of a carbon–carbon double bond by single bonds is energetically favored by using Tables 4E.2 and 4E.3 to calculate the reaction enthalpy for the conversion of ethene, C2H4, to ethane, C2H6. The reaction is![]()
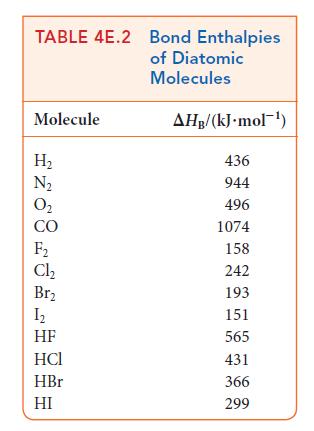
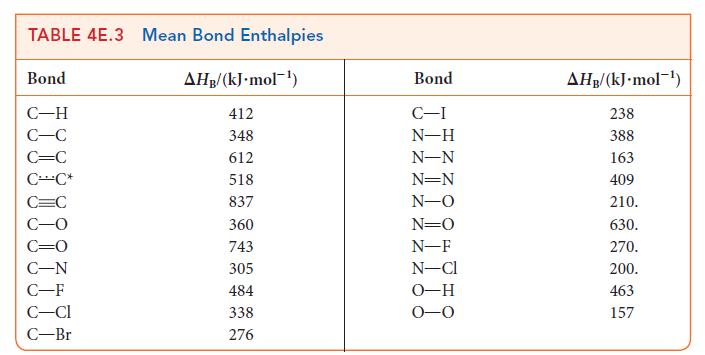
Transcribed Image Text:
H₂C=CH₂(g) + H₂(g) →CH3-CH3(g).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To calculate the reaction enthalpy for the conversion of ethene C2H4 to ethane C2H6 we need to consi...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane (C2H6), is a very important industrial chemical process. In principle,...
-
The replacement of a planing machine is being considered by the Reardorn Furniture Company. (There is an indefinite future need for this type of machine.) The best challenger will cost $30,000 for...
-
Although ab initio HF calculations fail in predicting molecular atomization energies, one can still use HF energies to estimate energy changes for certain types of reactions. Recall that good...
-
Assume that you are purchasing an investment and have decided to invest in a company in the digital phone business. You have narrowed the choice to Best Digital, Corp., and Every Zone, Inc., and have...
-
A job order cost sheet for Rolen Company is shown below. Instructions(a) On the basis of the foregoing data answer the following questions.(1) What was the balance in Work in Process Inventory on...
-
Sketch two cycles of the curve of a projection of the end of a radius on the y-axis. The radius is of length R and it is rotating counterclockwise about the origin at 2.00 rad/s. It starts at an...
-
You are the accountant of a company that is considering expanding its operations to a developing country. The CEO has asked for a report outlining what issues the company should consider from an ESG...
-
Creative Solutions designs Web pages for clients in the education sector. The companys job-costing system has a single direct cost category (Web-designing labor) and a single indirect cost pool...
-
1. How do price levels and the changes in price level determine a country's exch rate? 2. When are the spot and forward currency markets aligned with interest rates? 3. Are changes in exchange rates...
-
Suppose that you create two tiny systems consisting of three atoms each, and each atom can accept energy in quanta of the same magnitude. (a) How many distinguishable arrangements are there of two...
-
In an adiabatic process, no energy is transferred as heat. Indicate whether each of the following statements about an adiabatic process in a closed system is always true, always false, or true in...
-
Let \(Z\) have the standard normal distribution. (a) Find \(P\left(\begin{array}{lll}0 & Z & 65\end{array} ight)\). (b) Find \(P\left(\begin{array}{ll}Z & 54\end{array} ight)\). (c) Find...
-
Inez files an employment discrimination suit against Jiffy Delivery Service, under Title VII of the Civil Rights Act, based on Jiffys discharge of Inez. If Inez prevails in her prima facie case, one...
-
Meg wants to give Lori a pair of diamond earrings that Meg has in her safe-deposit box at First National Bank. Both Lori and Meg are signatories for that safe deposit box. Meg gives Lori the key to...
-
An automatic stay is a suspension of all judicial proceedings on the occurrence of an independent event. (True/False)
-
Benas files a bankruptcy petition under Chapter 7 to have his debts discharged. If Benas plan is approved, the debts most likely to be discharged include claims for a. back taxes accruing within...
-
When two secured parties have perfected security interests in the same collateral, generally the last to perfect has priority. (True/False)
-
How does education add to a nations capital stock?
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
The heat capacity of a bomb calorimeter was determined by burning 6.79 g of methane (energy of combustion = 802 kJ/ mol CH4) in the bomb. The temperature changed by 10.8oC. a. What is the heat...
-
The combustion of 0.1584 g benzoic acid increases the temperature of a bomb calorimeter by 2.54oC. Calculate the heat capacity of this calorimeter. (The energy re-leased by combustion of benzoic acid...
-
Combustion of table sugar produces CO2(g) and H2O(l). When 1.46 g of table sugar is combusted in a constant-volume (bomb) calorimeter, 24.00 kJ of heat is liberated. a. Assuming that table sugar is...
-
Sugarland Industries reported a net income of $790,750 on December 31, 2021. At the beginning of the year, the company had 540,000 common shares outstanding. On April 1, the company sold 29,400...
-
The functions f and g are defined as follows. 1(x) = 2431- g(x) = x+3x-4 2 X x +81 For each function, find the domain. Write each answer as an interval or union of intervals.
-
Convert to a logarithmic equation. 5-2 1 25

Study smarter with the SolutionInn App


