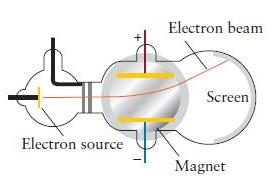
J. J. Thomson originally referred to the rays produced in his apparatus (Fig. 1A.2) as canal rays.
Question:
J. J. Thomson originally referred to the rays produced in his apparatus (Fig. 1A.2) as “canal rays.” The canal ray is deflected within the region between the poles of a magnet and strikes the phosphor screen. The ratio Q / m (where Q is the charge and m the mass) of the particles making up the canal rays is found to be 2.410 * 107 C·kg–1 . The cathode and anode of the apparatus are made of lithium, and the tube contains helium. Use the information inside the back cover to identify the particles (and their charges) that make up the canal rays. Explain your reasoning.
Figure 1A.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





