Many compounds used in the fragrance industry are derived from plant extracts. One step in identifying a
Question:
Many compounds used in the fragrance industry are derived from plant extracts. One step in identifying a desired compound is the determination of its molar mass. The volatile organic compound geraniol is a component of oil of roses. The density of the vapor at 260. °C and 103 Torr is 0.480 g · L–1. What is the molar mass of geraniol?
ANTICIPATE Because the compound is volatile, you should anticipate that it will have a moderately low molar mass.
PLAN List the information given and convert the temperature into an absolute value in kelvins. Then rearrange Eq. 8 into an expression for M, select a value of R with appropriate units, and substitute the data.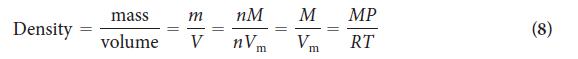
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





