Question:
One of the largest uses of electricity is in the production of aluminum by electrolysis of its oxide dissolved in molten cryolite (Na3AlF6). As an engineer, you might need to predict how much aluminum can be produced by this process. Find the mass of aluminum that can be produced in 1.00 day in an electrolytic cell operating continuously at 1.00 * 105 A. The cryolite does not react.
ANTICIPATE In this industrial-scale process, with a high current acting for a long time, you should expect to generate many kilograms of aluminum.
PLAN Use the first procedure in Toolbox 6O.1.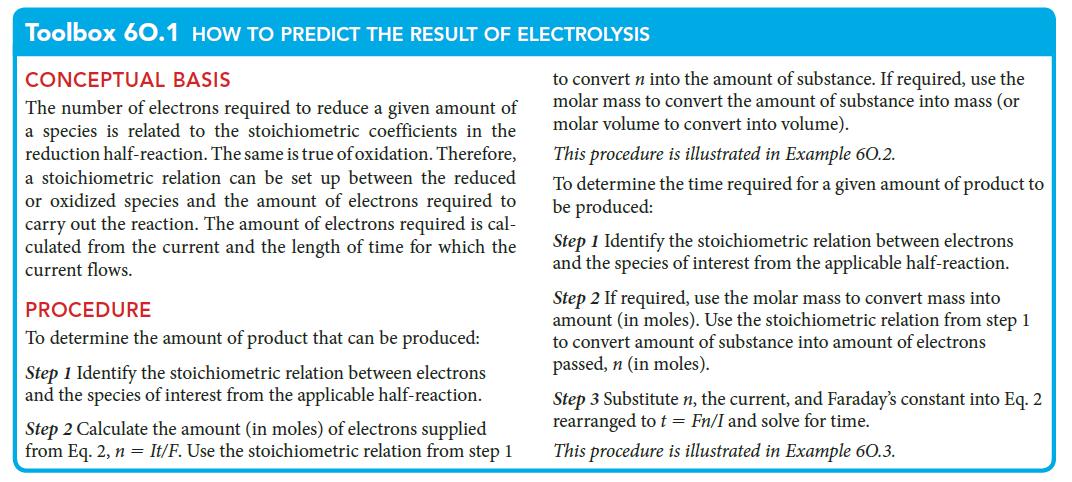
Transcribed Image Text:
Toolbox 60.1 HOW TO PREDICT THE RESULT OF ELECTROLYSIS
CONCEPTUAL BASIS
The number of electrons required to reduce a given amount of
a species is related to the stoichiometric coefficients in the
reduction half-reaction. The same is true of oxidation. Therefore,
a stoichiometric relation can be set up between the reduced
or oxidized species and the amount of electrons required to
carry out the reaction. The amount of electrons required is cal-
culated from the current and the length of time for which the
current flows.
PROCEDURE
To determine the amount of product that can be produced:
Step 1 Identify the stoichiometric relation between electrons
and the species of interest from the applicable half-reaction.
Step 2 Calculate the amount (in moles) of electrons supplied
from Eq. 2, n = It/F. Use the stoichiometric relation from step 1
to convert n into the amount of substance. If required, use the
molar mass to convert the amount of substance into mass (or
molar volume to convert into volume).
This procedure is illustrated in Example 60.2.
To determine the time required for a given amount of product to
be produced:
Step 1 Identify the stoichiometric relation between electrons
and the species of interest from the applicable half-reaction.
Step 2 If required, use the molar mass to convert mass into
amount (in moles). Use the stoichiometric relation from step 1
to convert amount of substance into amount of electrons
passed, n (in moles).
Step 3 Substitute n, the current, and Faraday's constant into Eq. 2
rearranged to t = Fn/I and solve for time.
This procedure is illustrated in Example 60.3.








