One of the reasons why thermonuclear weapons have to be serviced regularly is the nuclear decay of
Question:
One of the reasons why thermonuclear weapons have to be serviced regularly is the nuclear decay of the tritium, 3H, that they contain. Suppose you are monitoring the decay of tritium.
What mass of a tritium sample initially of mass 1.00 g will remain after 5.0 a (1 a = 1 year)? The decay constant of tritium is 0.0564 a–1.
ANTICIPATE The half-life of tritium (TABLE 10B.3) is 12.3 years, and so you should expect that more than half of the sample will remain after only 5 years.
PLAN The total mass of isotope in a sample is proportional to the number of nuclei of that isotope that the sample contains; therefore, the time dependence of the mass of a radioactive isotope follows the same radioactive decay law as the number of nuclides in the sample. That is, because m ∝ N, in place of Eq. 3 you can write m = m0e–kt, where m is the total mass of the radioactive isotope at time t and m0 is its initial mass.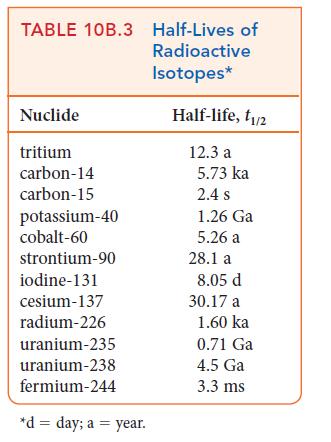
![]()
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





