Propane, C 3 H 8 , is a gas used as a fuel for outdoor grills and
Question:
Propane, C3H8, is a gas used as a fuel for outdoor grills and alternative-fuel vehicles. The enthalpy change for the synthesis of propane from its elements in their standard states is difficult to measure directly, but if you are interested in assessing the thermodynamic properties of its reactions, you need to know its value. Calculate the standard enthalpy of the reaction 3 C(gr) + 4 H2(g) → C3H8(g) from the following experimental data: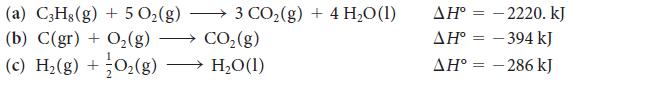
PLAN Use the procedure in Toolbox 4D.1 to combine the chemical equations in a way that yields the required overall equation.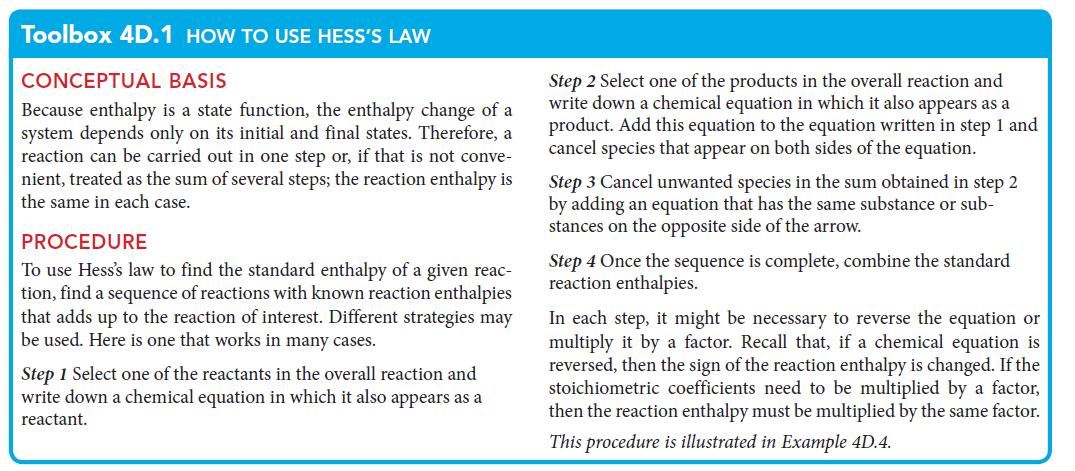
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





